the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Musculoskeletal infections associated with Nocardia species: a case series
Ryan B. Khodadadi
Jack W. McHugh
Supavit Chesdachai
Nancy L. Wengenack
Wendelyn Bosch
Maria Teresa Seville
Douglas R. Osmon
Elena Beam
Background: Nocardia is an uncommon pathogen that has been reported to infect musculoskeletal structures. However, studies are largely limited to case reports, and little is known regarding management and outcomes of these infections. Methods: We performed a multicenter retrospective cohort study of adults with culture-confirmed musculoskeletal Nocardia infections at three Mayo Clinic centers in Arizona, Florida, and Minnesota from November 2011 through April 2022. Results: Nine cases of Nocardia musculoskeletal infection were identified. Seven (78 %) occurred in men, and the median age was 57.3 years (range 32.6–79.0). Specific infections included native joint septic arthritis with or without associated osteomyelitis (N=3), hardware-associated infection (N=1), sternal osteomyelitis (N=1), pyomyositis (N=2), bursitis (N=1), and tenosynovitis (N=1). Three cases (33 %) were associated with disseminated disease, all three occurring in solid organ transplant recipients. Surgical intervention was performed in all but the bursitis case. Length of treatment varied from 21 d for tenosynovitis to 467 d for osteomyelitis. The 1-year mortality was 22 %, and all fatal cases involved disseminated disease. Conclusion: Patients with localized nocardiosis affecting musculoskeletal structures generally have good outcomes, as opposed to those with disseminated infection. Management often required operative intervention, with one patient experiencing recurrence within 1 year.
- Article
(565 KB) - Full-text XML
- BibTeX
- EndNote
Nocardia is a genus of aerobic filamentous Gram-positive bacteria that can cause infections in both immunocompetent and immunocompromised individuals (Traxler et al., 2022; Wilson, 2012). Clinical manifestations of nocardiosis are diverse, ranging from localized cutaneous lesions to disseminated infection with multiorgan involvement (Wilson, 2012). These infections can be challenging to diagnose and manage due to their indolent nature and the organism's potential resistance to various antimicrobial agents (Wilson, 2012).
Nocardia musculoskeletal infections including native joint septic arthritis, prosthetic joint infection, osteomyelitis, and tenosynovitis are rare, and there are sparse data describing associated clinical characteristics, management, and outcomes, with studies to date confined to case reports and small case series (Chaussade et al., 2015; Takamatsu et al., 2022). Given the limited information in the literature, we sought to review our institutional experience over the past decade with Nocardia musculoskeletal infections, outlining demographic, clinical, diagnostic, and treatment strategies as well as outcomes.
2.1 Study design
We performed a multicenter, retrospective cohort study of adults (≥18 years) with musculoskeletal infection from culture-confirmed Nocardia species obtained from clinical specimens at three Mayo Clinic centers in Arizona, Florida, and Minnesota from November 2011 to April 2022. Patients with culture growth of a Nocardia species were identified through our microbiology laboratory's internal registry. Patients were then manually screened for clinical infection as part of a separate study, and those with infection involving musculoskeletal structures were included in this study (Yetmar et al., 2023). Patients with a clinical syndrome compatible with musculoskeletal infection were identified for further analysis. Patients were excluded if they lacked clinical signs, symptoms, and/or radiographic changes consistent with infection; their age was less than 18 years on the date of diagnosis; or they lacked research authorization per Minnesota state statute (Minnesota Statutes, 2023).
Following the preliminary screening process, data including demographics; comorbidities included in the Charlson Comorbidity Index (CCI); race; ethnicity; and further details regarding lung disease, immunocompromised states including solid organ transplantation (SOT), hematopoietic stem cell transplant (HSCT), and immunosuppressant use were extracted from the electronic medical record. In addition, clinical, microbiologic, and treatment characteristics were collected manually from the electronic medical record and managed using REDCap electronic data capture tools (Harris et al., 2019, 2009). The clinical microbiology laboratory at Mayo Clinic in Rochester, Minnesota, received specimens for cultures, identification, and susceptibility testing from Mayo Clinic sites. Antimicrobial susceptibility testing was interpreted per guidelines of the Clinical and Laboratory Standards Institute (Clinical and Laboratory Standards Institute, 2018a, b). Additional information regarding Nocardia species identification and susceptibility testing for isolates in our reference microbiology laboratory at Mayo Clinic is described in a previous study (Yetmar et al., 2024). This study was reviewed and granted exempt status by our internal institutional review board (no. 22-008346).
2.2 Definitions
Musculoskeletal infection attributable to Nocardia species was defined as growth of Nocardia in culture with compatible signs, symptoms, and findings indicative of native or prosthetic musculoskeletal clinical infection. Date of diagnosis was defined as the date of the first culture acquisition that yielded Nocardia species. Localized disease was defined as isolation of Nocardia from the primary site of infection without evidence of additional sites of involvement. Disseminated infection was defined as the involvement of at least two non-contiguous organs. Immunocompromised status was defined as receipt of at least 20 mg d−1 of prednisone equivalent corticosteroid, receipt of other immunosuppressive medication, or HSCT within the preceding 100 d. Immunosuppressant use was assessed within the preceding 28 d of Nocardia diagnosis. Similarly, corticosteroid dosing was the most recent recorded dose in the 28 d prior to infection, converted to equivalent dosing of prednisone. Trimethoprim–sulfamethoxazole (TMP-SMX) prophylaxis was assessed on the date of initial presentation. Laboratory values were assessed on the date of diagnosis or the most recent prior measurement. Combination therapy was defined as receipt of at least two initial antibiotic agents. Active antibiotic agents were those that were initially used and later tested to be susceptible. Definitive antimicrobial therapy was defined as tailored antimicrobial therapy utilized for over half of a patient's treatment course. Progression was defined as clinical, symptomatic, or radiographic worsening of disease despite effective antimicrobial therapy for Nocardia musculoskeletal infection.
2.3 Statistical analysis
Descriptive statistics in this series are described as either a median value (range) for continuous variables or as a number (percentage) for categorical variables. The CCI was calculated as previously described (Charlson et al., 1994). All analyses were performed using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
From 511 patients with culture growth of Nocardia from a clinical specimen, 9 patients were diagnosed with Nocardia musculoskeletal infection. The median age was 57.3 years (range 32.6–79.0), with most patients being male (N=7, 78 %) and of white race (N=7, 78 %) and with a median CCI of 2.0 (range 0–6). The most common comorbidities observed were chronic kidney disease (N=4, 44 %), diabetes mellitus (N=3, 33 %), and prior SOT (N=4, 44 %), all of which were kidney transplants. Five patients were considered immunocompromised: four were receiving prednisone, tacrolimus, and mycophenolate following SOT, and one patient was receiving chronic prednisone for management of ulcerative colitis. Six patients had localized infection, while three patients presented with disseminated disease; all three with disseminated infection had a history of SOT. The type of musculoskeletal infection varied and included native joint septic arthritis with or without associated osteomyelitis (N=3, 33 %), ankle hardware-associated infection (N=1, 11 %), sternal osteomyelitis (N=1, 11 %), pyomyositis (N=2, 22 %), bursitis (N=1, 11 %), and tenosynovitis (N=1, 11 %). With respect to the hardware-associated infections, one patient developed infection of sternotomy wires 2 months following repair of an aortic aneurysm; the other developed purulent drainage at the site of pin placement 2 months following closed reduction and external fixation of an ankle fracture. There were no cases of prosthetic joint infection. The most common Nocardia species isolated included N. cyriacigeorgica (N=2, 22 %), N. nova (N=2, 22 %), and N. farcinica (N=2, 22 %). Additional cohort characteristics and case details are outlined in Table 1.
Table 1Case details of nine patients with Nocardia musculoskeletal infection.
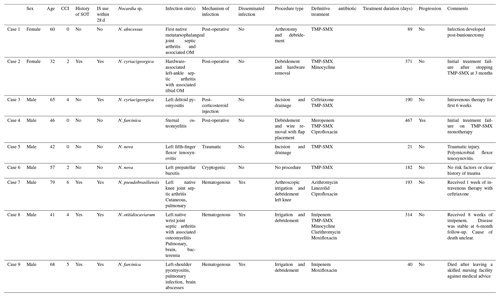
CCI: Charlson Comorbidity Index. SOT: solid organ transplant. IS: immunosuppressant. TMP-SMX: trimethoprim–sulfamethoxazole. OM: osteomyelitis.
Antimicrobial susceptibility results are shown in Table 2, which were available for all nine isolates. All isolates were susceptible to amikacin (N=9, 100 %) and linezolid (N=9, 100 %), and most isolates were susceptible to TMP-SMX (N=7, 78 %). Otherwise, susceptibility to other antimicrobial agents varied, with imipenem being the most effective among beta-lactams. For treatment, most patients received TMP-SMX (N=7, 78 %), followed by imipenem (N=4, 44 %) and linezolid (N=3, 33 %). The median number of initial agents was two (range one to three), with a median length of therapy of 190 d (range 21–467). Of the four cases occurring in SOT, mycophenolate was held in three cases where there was evidence of disseminated disease, and tacrolimus and prednisone were continued. In combination with antimicrobial therapy, surgical intervention was performed in all cases but one for surgical source control. For localized infection, a single surgery was effective for clinical cure in four of six cases (67 %). The 1-year mortality was 22 %, occurring at 42 and 327 d after Nocardia diagnosis, respectively. Median post-treatment follow-up was 512 d (range: 160–3451). Only two patients received secondary prophylaxis with either TMP-SMX or doxycycline for confirmed susceptible isolates.
Following initial treatment, progression was observed in one case of TMP-SMX-susceptible N. farcinica sternal osteomyelitis. This occurred on TMP-SMX monotherapy, likely due to incomplete initial surgical debridement, as the patient ultimately required serial debridement procedures followed by sternal wire removal and flap placement. In the case of hardware-associated left-ankle septic arthritis, the patient developed purulent drainage from external pins 3 months post-closed reduction of a left calcaneal fracture. Hardware was removed, and 12 months of treatment was recommended. TMP-SMX monotherapy was abruptly discontinued by the patient after 3 months with recrudescence of infection due to incomplete treatment confirmed on operative cultures at the time of repeat surgery. Following additional operative intervention to achieve surgical source control and tailoring of empiric therapy to N. abscessus susceptibilities, the patient completed 281 additional days of treatment with TMP-SMX and minocycline.
Musculoskeletal infections caused by Nocardia species pose significant challenges in clinical management due to their rare occurrence, variable clinical presentations, and the need for prolonged antibiotic therapy. In this study, we reviewed our institutional experience and investigated clinical characteristics, management strategies, and outcomes of musculoskeletal Nocardia infections to enhance our understanding and further inform clinical practice of this rare condition.
Our findings revealed a low incidence of musculoskeletal Nocardia infections amongst all cases of nocardiosis at our institution over the past decade, consistent with previous reports (Takamatsu et al., 2022; Chaussade et al., 2015). In 2022, a literature review identified 37 reported cases of septic arthritis from Nocardia species, 5 of which were prosthetic joint infections (Fazili et al., 2022). Other Nocardia musculoskeletal infections such as osteomyelitis, pyomyositis, and tenosynovitis have been confined to case reports. The rarity of these infections emphasizes the importance of a high index of suspicion, particularly in patients with underlying immunocompromising conditions or a recent history of trauma or surgery.
Clinical manifestations of musculoskeletal Nocardia infection varied widely, ranging from localized infection secondary to trauma to deeper involvement of musculoskeletal structures as a consequence of dissemination in immunocompromised hosts (Wilson, 2012). Optimal management of musculoskeletal Nocardia infections requires a multidisciplinary approach, involving orthopedic surgeons and infectious diseases specialists (Wilson, 2012). Surgical intervention, such as drainage of abscesses and debridement of infected tissues, is often necessary, particularly in cases of extensive involvement or implant-associated infections. Indeed, nearly all patients in our cohort required a surgical procedure, with one requiring further surgeries due to progression after an incomplete debridement. Our study reaffirms the importance of timely and aggressive surgical debridement to control these infections and prevent disease progression.
In combination with surgical source control, prolonged antimicrobial therapy remains a cornerstone of treatment for musculoskeletal Nocardia infections. However, selecting the most appropriate antibiotic regimen upfront remains challenging due to the limited clinical data on musculoskeletal Nocardia, bone penetration of available antimicrobials, long turnaround times for species identification, and variable susceptibility patterns among Nocardia species. Our study identified TMP-SMX as the most commonly used antibiotic agent, either as monotherapy or in combination with other antimicrobials depending on extent of involvement.
The optimal duration of treatment for musculoskeletal Nocardia infections is overall poorly understood, and individualized treatment takes into consideration a multitude of factors including the immune status of the affected patient, extent of involvement, and whether surgical source control has been effectively achieved as well as response to initial therapy (Restrepo and Clark, 2019; Wilson, 2012). While treatment duration for nocardiosis remains controversial, some studies have suggested a longer duration of treatment may be associated with fewer episodes of post-treatment recurrence (Yetmar et al., 2024; Tashiro et al., 2018). This risk likely is affected by the site of infection, and most patients included in this cohort of musculoskeletal infections were treated with prolonged antibiotics. One patient did have an early recurrence after a relatively brief course of treatment (90 d) though ultimately had a good outcome after restarting treatment for a longer period. Two patients also utilized post-treatment secondary prophylaxis, though data are limited overall regarding the use of prophylaxis for nocardiosis (Yetmar et al., 2024; Passerini et al., 2024). Long-term antibiotic suppression is commonly utilized in patients with musculoskeletal infection, particularly those with retained prosthetic material (Cortes-Penfield et al., 2023). Further study is needed to evaluate treatment duration and prophylaxis for nocardiosis, though close follow-up and clinical assessment for evaluation of treatment response and complications during and after therapy are crucial.
Favorable clinical responses were observed in the majority of patients following appropriate surgical and medical management. However, a subset of patients, in particular one SOT patient, experienced treatment failure with progression, highlighting the importance of long-term follow-up and surveillance to determine if failure is related to antibiotic therapy, lack of surgical source control, and profound immunosuppression (Restrepo and Clark, 2019). Further research is warranted to elucidate the prognostic factors associated with poor outcomes and refine treatment algorithms for musculoskeletal Nocardia infections.
Strengths of this study include the number of included cases, as most prior studies are case reports. We also had susceptibility data for all isolates as well as robust clinical, diagnostic, and treatment details available in the electronic medical record at our institution. However, there are several limitations worth noting. While large for this topic, there were still relatively few cases, which may limit the generalizability of this study's findings and precluded more formal statistical analyses. Further, the considerable diversity in underlying clinical syndromes precludes the formulation of overarching conclusions. Additionally, this study was retrospective and observational in nature and prone to unmeasured sources of bias. In addition, there were only a few patients with disseminated disease, and overall, there was a lack of uniform follow-up.
In conclusion, musculoskeletal Nocardia infections represent a rare but clinically significant entity that requires prompt recognition and aggressive management. Our study provides valuable insights into the epidemiology, clinical characteristics, and treatment outcomes of these infections, guiding clinicians in their approach to diagnosis and management. Collaborative efforts across specialties, continued surveillance of antimicrobial resistance patterns, and further research are crucial for improving patient outcomes and reducing the burden of musculoskeletal Nocardia infections.
The dataset is available upon request.
Conceptualization of manuscript: RBK, JWM, ZAY, EB. Data collection: RBK, ZAY, JWM, SC. Data analysis: ZAY. Writing (original draft): RBK, ZAY, JWM. Reviewing and editing: RBK, ZAY, JWM, SC, NLW, WB, MTS, DRO, EB. Supervision: ZAY.
The contact author has declared that none of the authors has any competing interests.
This study was deemed exempt by the Institutional Review Board of Mayo Clinic (IRB ID 22-008346).
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This research has been supported by the National Center for Advancing Translational Sciences (grant no. UL1 TR002377).
This paper was edited by Marjan Wouthuyzen-Bakker and reviewed by two anonymous referees.
Charlson, M., Szatrowski, T. P., Peterson, J., and Gold, J.: Validation of a combined comorbidity index, J. Clin. Epidemiol., 47, 1245–1251, https://doi.org/10.1016/0895-4356(94)90129-5, 1994.
Chaussade, H., Lebeaux, D., Gras, G., Catherinot, E., Rammaert, B., Poiree, S., Lecuyer, H., Zeller, V., Bernard, L., and Lortholary, O.: Nocardia Arthritis: 3 Cases and Literature Review, Medicine, 94, e1671, https://doi.org/10.1097/md.0000000000001671, 2015.
Clinical and Laboratory Standards Institute: Susceptibility testing of mycobacteria, Nocardia spp, and other aerobic actinomycetes, 3rd edn., Wayne: Clinical and Laboratory Standards Institute, CLSI guideline M24, 2018a.
Clinical and Laboratory Standards Institute: Performance standards for susceptibility testing of mycobacteria, Nocardia spp, and other aerobic actinomycetes, Wayne: Clinical and Laboratory Standards Institute, CLSI guideline M62, 2018b.
Cortes-Penfield, N., Krsak, M., Damioli, L., Henry, M., Seidelman, J., Hewlett, A., and Certain, L.: How We Approach Suppressive Antibiotic Therapy Following Debridement, Antibiotics, and Implant Retention for Prosthetic Joint Infection, Clin. Infect. Dis., 78, 188–198, https://doi.org/10.1093/cid/ciad484, 2023.
Fazili, T., Bansal, E., Garner, D., Bajwa, V., and Vasudeva, S.: Septic arthritis due to Nocardia: Case report and literature review, Am. J. Med. Sci., 364, 88–91, https://doi.org/10.1016/j.amjms.2022.01.012, 2022.
Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., and Conde, J. G.: Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform., 42, 377–381, https://doi.org/10.1016/j.jbi.2008.08.010, 2009.
Harris, P. A., Taylor, R., Minor, B. L., Elliott, V., Fernandez, M., O'Neal, L., McLeod, L., Delacqua, G., Delacqua, F., Kirby, J., and Duda, S. N.: The REDCap consortium: Building an international community of software platform partners, J. Biomed. Inform., 95, 103208, https://doi.org/10.1016/j.jbi.2019.103208, 2019.
Minnesota Statutes: https://www.revisor.mn.gov/statutes/cite/144.295 (last access: 9 August 2024), 2023.
Passerini, M., Nayfeh, T., Yetmar, Z. A., Coussement, J., Goodlet, K. J., Lebeaux, D., Gori, A., Mahmood, M., Temesgen, Z., and Murad, M. H.: Trimethoprim-sulfamethoxazole significantly reduces the risk of nocardiosis in solid organ transplant recipients: systematic review and individual patient data meta-analysis, Clin. Microbiol. Infect., 30, 170–177, https://doi.org/10.1016/j.cmi.2023.10.008, 2024.
Restrepo, A. and Clark, N. M.: Nocardia infections in solid organ transplantation: Guidelines from the Infectious Diseases Community of Practice of the American Society of Transplantation, Clin. Transplant., 33, e13509, https://doi.org/10.1111/ctr.13509, 2019.
Takamatsu, A., Yaguchi, T., Tagashira, Y., Watanabe, A., and Honda, H.: Nocardiosis in Japan: a Multicentric Retrospective Cohort Study, Antimicrobial Agents and Chemotherapy, 66, e01890-01821, https://doi.org/10.1128/aac.01890-21, 2022.
Tashiro, H., Takahashi, K., Kusaba, K., Tanaka, M., Komiya, K., Nakamura, T., Aoki, Y., Kimura, S., and Sueoka-Aragane, N.: Relationship between the duration of trimethoprim/sulfamethoxazole treatment and the clinical outcome of pulmonary nocardiosis, Respir. Investig., 56, 166–172, https://doi.org/10.1016/j.resinv.2017.11.008, 2018.
Traxler, R. M., Bell, M. E., Lasker, B., Headd, B., Shieh, W. J., and McQuiston, J. R.: Updated Review on Nocardia Species: 2006–2021, Clin. Microbiol. Rev., 35, e0002721, https://doi.org/10.1128/cmr.00027-21, 2022.
Wilson, J. W.: Nocardiosis: updates and clinical overview, Mayo Clin. Proc., 87, 403–407, https://doi.org/10.1016/j.mayocp.2011.11.016, 2012.
Yetmar, Z. A., Khodadadi, R. B., Chesdachai, S., McHugh, J. W., Challener, D. W., Wengenack, N. L., Bosch, W., Seville, M. T., and Beam, E.: Mortality After Nocardiosis: Risk Factors and Evaluation of Disseminated Infection, Open Forum Infect. Dis., 10, ofad409, https://doi.org/10.1093/ofid/ofad409, 2023.
Yetmar, Z. A., Khodadadi, R. B., Chesdachai, S., McHugh, J. W., Challener, D. W., Wengenack, N. L., Bosch, W., Seville, M. T., and Beam, E.: Epidemiology, Timing, and Secondary Prophylaxis of Recurrent Nocardiosis, Open Forum Infect. Dis., 11, ofae122, https://doi.org/10.1093/ofid/ofae122, 2024.





