the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Diagnosis of vertebral osteomyelitis
Julian Maamari
Aaron J. Tande
Felix Diehn
Don Bambino Geno Tai
Elie F. Berbari
Native vertebral osteomyelitis (NVO) is a potentially fatal infection which has seen a gradual increase in its incidence over the past decades. The infection is insidious, presenting with symptoms of back pain. Fever is present in about 60 % of patients. Prompt diagnosis of NVO is important to prevent the development of complications. Numerous laboratory and imaging tools can be deployed to accurately establish the diagnosis. Imaging techniques such as magnetic resonance, nuclear imaging, and computed tomography are essential in diagnosing NVO but can also be useful in image-guided biopsies. Laboratory tools include routine blood tests, inflammatory markers, and routine culture techniques of aspirated specimens. Recent advances in molecular techniques can assist in identifying offending pathogen(s). In this review, we detail the arsenal of techniques that can be utilized to reach a diagnosis of NVO.
- Article
(405 KB) - Full-text XML
- BibTeX
- EndNote
Native vertebral osteomyelitis (NVO), also termed spondylodiscitis, is a potentially fatal condition that constitutes roughly 3 %–5 % of all osteomyelitis cases (Sobottke et al., 2008). Its incidence has increased from 2.9 cases to 5.4 cases per 100 000 people in the United States between 1998 and 2013, owing partly to a demographic shift towards an older and immunocompromised population (Issa et al., 2018). Due to relative rarity and nonspecific symptoms, delays in the diagnosis of NVO still happen despite the expanding use and availability of magnetic resonance imaging (MRI). A prospective study on NVO found a mean diagnostic delay of 45.5 d from the onset of symptoms (range 2–280 d). Other studies have suggested even longer delays, with variations attributed to the causative organism (Jean et al., 2017).
NVO is most commonly the result of hematogenous seeding of the avascular disc. Other causes include contiguous spread and direct inoculation during surgery (Zimmerli, 2010). The most common symptom at the time of presentation is back pain (Mylona et al., 2009). Although highly sensitive (86 %), this symptom lacks specificity, particularly among older adults. Other symptoms of NVO, such as fever (60 %) and neurologic deficits, including radiculopathy, urinary retention, limb weakness, paralysis, dysesthesia, or sensory loss (34 %) are less common (Mylona et al., 2009). Routinely performed inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are sensitive but also lack specificity (Zimmerli, 2010). Therefore, maintaining a high index of suspicion is crucial for establishing the diagnosis of NVO.
There are no widely agreed upon diagnostic criteria for diagnosing NVO, particularly in cases with negative blood and biopsy cultures. Instead, NVO is diagnosed through a compatible overall clinical picture, combined with suggestive imaging and laboratory findings (Berbari et al., 2015). Early diagnosis and treatment are essential to decrease the risk of complications, neurologic deficits, and mortality (Gupta et al., 2014). This review summarizes the literature on the various diagnostic modalities employed to diagnose NVO.
Inflammatory biomarkers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), are the most well-studied screening tests for NVO in the setting of back pain. (Berbari et al., 2015). Both markers have been found to have a sensitivity in the range of 94 %–100 %, particularly when used in combination (Berbari et al., 2015). Logistic regression of a cohort of 72 patients with suspected NVO undergoing image-guided biopsy revealed that the combination of ESR, CRP, and the presence of fever has the highest area under the curve (AUC = 0.72) for predicting a diagnosis of NVO. Enhancement of the predictive yield was observed when MRI results were factored in (Kihira et al., 2020). ESR is typically more elevated in common bacterial NVO than in tuberculous NVO, with more than 91 % of NVO patients having an initial ESR value > 50 mm h−1 (Waheed et al., 2019). One study suggested that using a score that encompasses CRP, pain severity grading, and imaging findings may be a useful tool in the diagnosis, treatment, and follow-up of patients with NVO (Homagk et al., 2019). CRP and ESR may also help predict relapse following treatment (Ahn et al., 2020; McHenry et al., 2002; Chiang et al., 2019; Carragee et al., 1997). Serum white blood cell (WBC) count has low sensitivity and specificity. Leukocytosis is often absent or only mildly elevated (An and Seldomridge, 2006). Apart from CRP and ESR, no novel biomarkers have paved their way into clinical practice in recent decades. Efforts to identify other reliable biomarkers are warranted, especially in the setting of partially treated NVO or infection with an indolent organism.
Although MRI is the preferred imaging modality for the diagnosis of NVO, we recommend obtaining a plain radiograph of the spine as an initial test (Diehn, 2012). Plain radiography has low sensitivity at the early stages of the disease, but it may help identify other causes of back pain and establish spinal enumeration. Subtle findings, such as loss of definition, erosions, and irregularity of the vertebral end plates, typically lag behind the disease, only appearing 2 to 8 weeks after the onset of symptoms (Govender, 2005). If present on a prior radiograph, the disappearance of a previously seen degenerative gas in the disc space (disc space vacuum phenomenon) can be suggestive of NVO, particularly if it is associated with disc space widening and/or end plate erosions.
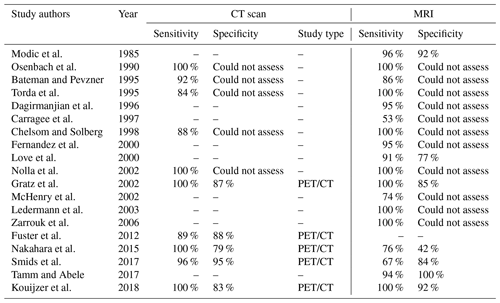
MRI is the preferred imaging modality for diagnosing NVO (Diehn, 2012). The sensitivity, specificity, and accuracy of MRI in diagnosing NVO are estimated at 97 %, 92 %, and 94 %, respectively (Table 1; Modic et al., 1985). MRI should ideally be performed with intravenous gadolinium contrast. It increases the sensitivity and specificity of the MRI, including a better depiction of a possible extension of infection to the epidural and paravertebral spaces. T2-weighted and post-contrast T1-weighted images should be acquired with fat suppression. A hallmark of the disease is the presence of marrow-replacing signal abnormalities, seen best on T1-weighted non-contrast images. The normal marrow is hyperintense compared with the intervertebral discs, whereas abnormal marrow is relatively hypointense. Such an abnormal marrow signal on T1-weighted images typically correlates with T2 hyperintensity, which is best seen on fat-suppressed T2-weighted images, and enhancement, which is best seen on post-contrast fat-suppressed T1-weighted images. (Berbari et al., 2015; Prodi et al., 2016). The disc itself may also be abnormally T2 hyperintense or enhancing. Although MRI can detect bone marrow edema as early as 48 h after disease onset, early findings may be nonspecific or atypical; the primary confounders are active sub-end plate degenerative changes (so-called Modic type I changes). In these patients, an MRI can be repeated in 2–4 weeks to further evaluate the diagnosis of NVO (Kamiya et al., 2019). The inclusion of diffusion-weighted imaging on MRI is sometimes used to help increase the specificity of bone marrow edema for NVO (Patel et al., 2014). Routine follow-up MRI for clinically improving patients on treatment is unnecessary, as the imaging resolution can lag behind clinical improvement (Kowalski et al., 2007). At times, MRI may provide clues to the causative organism (Hong et al., 2009); for example, a multilevel process with subligamentous extension and prominent paraspinal component with relative sparing of the disc spaces may suggest Mycobacterium tuberculosis.
Computed tomography (CT) is another imaging technique that can help diagnose NVO (Table 1). CT can be beneficial in cases where Modic type I changes are a primary consideration based on MRI, and the clinical findings do not strongly suggest an infection. In such patients, the absence of end plate cortical erosive changes makes NVO is less likely. CT is superior to MRI with respect to the evaluation of cortical bone and depicting the disc space vacuum phenomenon. In rare cases, gas in the disc is related to a gas-forming organism or other anatomic abnormality, such as a fistula with the gastrointestinal tract (Diehn, 2012).
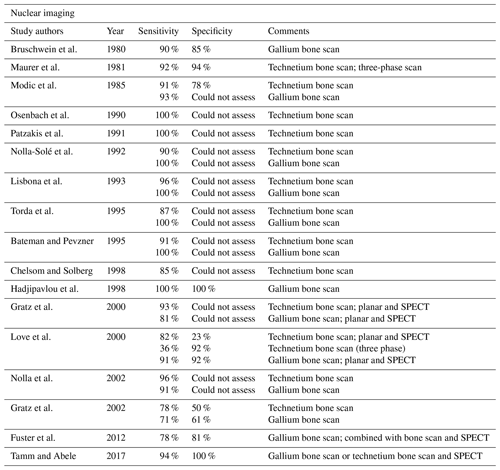
Nuclear imaging techniques have also been employed successfully to diagnose NVO (Prodi et al., 2016). They may be the alternative in cases with severe degenerative arthritis, potential neuropathic arthropathy (Charcot spine), or when MRI is contraindicated (Love et al., 2000). Scintigraphy with single-photon emission computed tomography (SPECT) using Technetium-99m (99mTc) and Gallium-67 (67Ga) tracers are the most widely used methods. Studies showed that 99mTc scintigraphy has high sensitivity (90 %) but moderate specificity. Combining the two techniques increases the sensitivity, with some studies suggesting that 67Ga or 99mTc scanning alone may be insufficient to diagnose NVO. These studies demonstrated that these techniques were equivalent to MRI (Modic et al., 1985; Maurer et al., 1981; Hadjipavlou et al., 1998; Tamm and Abele, 2017). Combining both techniques is the standard of care if used in place of MRI (Tamm and Abele, 2017). Tracer uptake that is greater or anatomically discordant on the gallium (inflammation detecting) than on the technetium (metabolism detecting) portion of the combined nuclear medicine study is the finding which most strongly and accurately suggests NVO (Diehn, 2012). Positron emission tomography–computed tomography (PET/CT) has also been evaluated for the diagnosis of NVO. The literature suggests that the technique may be more accurate than combined 67Ga and 99mTc scans with similar accuracy compared to MRI (Fuster et al., 2012; Kouijzer et al., 2018). The advantages of PET/CT include its superior spatial resolution and the better detection of metastatic infection. In addition, a CT scan itself may hold an advantage in detecting sequestra, cloacas, involucra, or intraosseous gas, which may form in chronic NVO (Pineda et al., 2009); however, MRI remains a superior imaging modality in detecting small intraspinal (e.g., epidural) and paraspinal abscesses (Tables 1, 2; Fuster et al., 2012; Kouijzer et al., 2018).
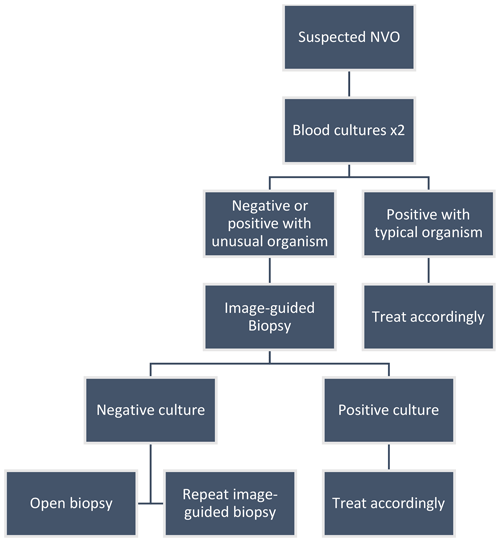
Optimal management relies on the isolation of the causative organism. The initial step is collecting bacterial blood cultures, which are positive in approximately 58 % of cases (range 30 %–78 %) (Mylona et al., 2009; Zimmerli, 2010). The Infectious Diseases Society of America (IDSA) guidelines recommend obtaining two sets of bacterial blood cultures (aerobic and anaerobic) in patients with suspected NVO. When positive, blood cultures may obviate the need for biopsies (Berbari et al., 2015). However, the yield of blood cultures may be affected by previous antibiotic therapy. Most cases of NVO that result from hematogenous seeding are monomicrobial. Other causes associated with contiguous spread or direct inoculation tend to be more polymicrobial (Mavrogenis et al., 2017). If infection with a typical organism – i.e., Staphylococcus aureus complex, Staphylococcus lugdunensis, or Brucella species – is established with blood cultures or serologic testing, no further investigation may be necessary (Berbari et al., 2015). An image-guided biopsy is warranted when blood cultures or serologic testing does not establish the microbiologic diagnosis (Berbari et al., 2015). The two most widely recognized methods are image-guided percutaneous biopsy and open biopsy (McNamara et al., 2017). Percutaneous biopsies and aspirations are typically guided by CT or fluoroscopy (Kim et al., 2013). These sampling procedures can target the bone, disc, and adjacent infected spinal sites such as facet joints or paraspinal soft tissues, including abscesses. Intraspinal sampling (e.g., of epidural abscesses) can be performed if there are accessible dorsal, relatively large components to the intraspinal collections. Otherwise, it is not routinely performed due to the risk of inadvertent dural puncture. Percutaneous biopsies have variable microbiologic yields of 30.4 %–91 % (Chew and Kline, 2001; Pupaibool et al., 2015). Two meta-analyses calculated the cumulative yield between 48 % and 52 %, significantly lower than the 76 % yield in open biopsies (McNamara et al., 2017; Pupaibool et al., 2015). Factors that may increase the yield of the image-guided procedure include an elevated CRP; the use of a lower-gauge needle, increased number of specimens obtained; and, if present, the aspiration of a fluid collection (Husseini et al., 2020; Gras et al., 2014). The impact of prior antibiotic use on image-guided specimens' culture yield remains uncertain, and the findings of existing studies are conflicting: some studies indicate that prior antimicrobial therapy negatively impacted the yield, whereas some indicate no effect. The studies were limited in their retrospective design, sample size, and selection bias (Wong et al., 2021). If the initial biopsy is nondiagnostic, a second percutaneous biopsy may be warranted, although the exact increased yield is unclear (Gras et al., 2014). A repeat biopsy should be delayed at least 3 d after the initial biopsy, at which time the majority of positive cultures from the first should have resulted (Yeh et al., 2020). Alternatively, when the first image-guided biopsy is negative, it is reasonable to proceed with an open biopsy as the next step (Fig. 1; Berbari et al., 2015).
Specimens should be sent for both microbiologic and histopathologic examination. Histopathology reveals the presence of acute inflammatory cells in 69 %–95 % of cases (Iwata et al., 2019; Heyer et al., 2012). Biopsy specimens should be sent for aerobic and anaerobic bacterial cultures. Fungal, zoonotic, and mycobacterial etiologies should be considered in patients with culture-negative NVO, immunocompromising conditions, or risk factors such as living in endemic areas (Berbari et al., 2015; Mavrogenis et al., 2017). Patients who are immunocompromised are particularly susceptible to non-endemic fungal organisms such as Candida spp., Aspergillus spp., and Cryptococcus neoformans (Hong et al., 2009; Salaffi et al., 2021). C. albicans is responsible for more than half of candidal NVO cases, although Nakaseomyces glabrata – previously C. glabrata – is also becoming more common. Modern bacterial blood culture techniques are capable of identifying Candida species. Aspergillus NVO may mimic tuberculous NVO particularly when the intervertebral disc is spared, with the most commonly isolated species being A. fumigatus (Salaffi et al., 2021). In patients at risk of fungal infections, fungal serologies, antigen detection assays, and fungal blood cultures may also be useful (Berbari et al., 2015). Proving a diagnosis of NVO in these cases requires documenting a positive culture or histology result, a clinical picture compatible with NVO, and radiologic evidence of the infection (De Pauw et al., 2008). Coccidioidomycosis and blastomycosis are the most important endemic fungal infections that may cause NVO. C. immitis localizes to the bone in more than 50 % of diffuse cases, whereas bone involvement is noted in 14 %–60 % of diffuse blastomycosis, with the spine being the most commonly involved location (Salaffi et al., 2021; Hong et al., 2009). However, serologic testing for Coccidioides and Blastomyces species may be considered if epidemiologic factors exist (Berbari et al., 2015).
For Brucella NVO, serologies and Brucella blood cultures are diagnostic tests of choice. A cutoff of > 1 : 160 for Brucella antibodies or > 1 : 320 for the Coombs test is considered positive (Berbari et al., 2015; Tali et al., 2015). Pott's disease (tuberculous NVO) should be suspected among patients with known or suspected tuberculosis at another site or living in areas endemic for TB. In these cases, a purified protein derivative test or an interferon-γ release assay could be helpful due to these tests' high negative predictive value (NPV) (Berbari et al., 2015; Colmenero et al., 2013). Lastly, a parasitic infection – although rare – may be present in some cases but with more unusual pathogens. Echinococcus species are parasites with a propensity to infect the bone and cause vertebral hydatid disease (Salaffi et al., 2021).
Among patients in whom targeted investigations, blood cultures, and biopsy cultures are negative, the results of other microbiologic data that correlate with the timing of onset of symptoms, such as preceding urine cultures or known colonization with resistant pathogens, can also be considered when formulating an empiric antimicrobial therapy program (Chenoweth et al., 2018). Transesophageal echocardiography (TEE) may be considered in selected NVO patients to rule out endocarditis as a source of infection (Behmanesh et al., 2019).
Some conditions mimic the presentation of NVO. Typical mimickers can be categorized into degenerative, inflammatory, metabolic/deposition, pseudoarthrosis, malignancy, or treatment related, including radiotherapy (Morales, 2018; Salaffi et al., 2021). These conditions are summarized in Table 3. Differentiating NVO from these entities is of utmost importance given the therapeutic and prognostic implications. The role of additional imaging, careful evaluation of images, and histopathology is invaluable in these cases (Morales, 2018). The “claw sign,” seen on diffusion-weighted MRI, was shown to be highly suggestive of Modic type 1 degenerative changes (Patel et al., 2014). In addition, the predominant involvement of one end plate also makes degenerative causes such as Schmorl's nodes more likely than an infectious etiology (Morales, 2018). When considering an inflammatory cause, clues such as multilevel involvement, subluxations, involvement of the posterior elements, and the detection of sacroiliitis would favor the diagnosis of a spondyloarthropathy (Morales, 2018).
Table 3Mimickers of native vertebral osteomyelitis.
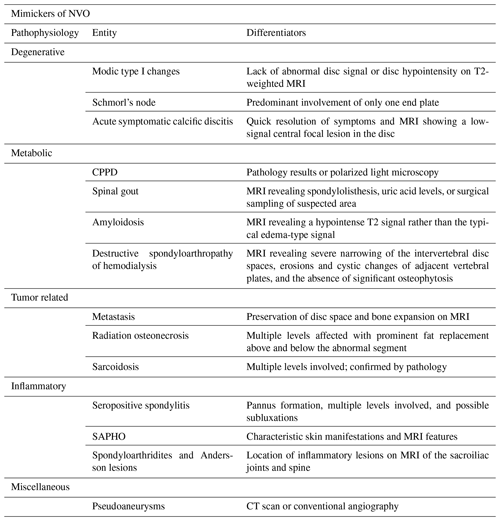
CPPD represents calcium pyrophosphate dihydrate crystal deposition disease, and SAPHO represents synovitis, acne, pustulosis, hyperostosis, osteitis syndrome.
Another example is highlighted in cases of sacral osteomyelitis, where MRI cannot easily distinguish bone remodeling/fibrosis from osteomyelitis, leading to a specificity as low as 22 % despite a high sensitivity. A bone biopsy after debridement is necessary to establish the diagnosis of NVO (Wong et al., 2019). Neuropathic arthropathy (Charcot spine) can also mimic NVO; the presence of exuberant osseous debris on especially CT images can be helpful in establishing this diagnosis.
Novel tools for imaging and microbiologic diagnosis of NVO have emerged. MRI-guided biopsies have long been limited by the resolution offered (often 0.5 T or less). Low-tesla open-magnet MRI scanners have been shown to have an 86 % sensitivity with a 100 % specificity for MRI-guided biopsies (Carrino et al., 2007). Recent advances in MRI have led to even more promising results for these biopsies, owing to the improved resolution and signal-to-noise ratios of modern scanners. However, the efficacy of this method has not been adequately examined, as opposed to CT-guided techniques (Wu et al., 2012).
Novel molecular diagnostic techniques have also garnered significant interest. Studies investigating the use of 16S ribosomal RNA (rRNA) gene polymerase chain reaction (PCR) on suspected cases of NVO have supported its potential role in improving accuracy and time to diagnosis (Sheikh et al., 2017; Choe et al., 2014). These methods complement standard microbiologic methods, particularly difficult to identify microorganisms. Although they lack information on antimicrobial susceptibility, microorganism identification will guide antibiotic therapy (Zimmerli, 2010; Choe et al., 2014; Lecouvet et al., 2004). GeneXpert PCR for spinal tuberculosis is highly sensitive and specific (> 95 %), with the ability to detect multidrug-resistant tuberculosis (Held et al., 2014).
Metagenomic next-generation sequencing (mNGS) is another novel technique that has proven helpful in identifying various infectious agents. This technology allows the high-throughput sequencing of billions of nucleic acid fragments in a manner much more efficient than the classic Sanger sequencing technique (Lefterova et al., 2015). It carries the benefit of allowing timely detection of one or more pathogens simultaneously, particularly when fastidious, slow-growing or atypical bacteria are implicated (Salipante et al., 2013; Lefterova et al., 2015). Unlike culture methods, mNGS can often determine resistance genes to the molecular levels (Morcrette et al., 2018). The utility of mNGS in osteoarticular infections has been validated in a prospective study conducted on 130 samples of fluid or tissue. The study revealed a positive mNGS rate of 88.5 % compared with 69.2 % associated with culture. However, 16 pathogens isolated in cultures were missed by mNGS in the study due to various reasons. Thus, the technique is only recommended as a complementary study to culture until it is further optimized (Huang et al., 2020). Metagenomic studies are becoming more cost-effective and accurate with time. As reference databases are improved and more pathogen genomes are sequenced, its use is expected to increase and provide more utility, particularly for osteoarticular infections such as NVO (Lefterova et al., 2015; Morcrette et al., 2018).
Many institutions have recently adopted the inoculation of biopsy specimens in blood culture bottles to enhance the recovery of microorganisms. A study using the BACTEC™ 9050 culture bottles (Becton, Dickinson and Company, NJ, USA) for these specimens revealed yields similar to those previously reported in the literature (Pandita et al., 2019). It remains to be seen whether the use of these techniques will optimize the yield of NVO biopsies.
As the methods of NVO diagnosis evolve, early detection continues to be the primary goal. A high index of suspicion can direct a clinician's approach, allowing targeted testing and management. Optimal management of NVO includes accurate identification of the causative agent and treatment with targeted antimicrobial therapy followed by long-term remission. Therefore, we must conduct studies to optimize routinely used techniques, such as image-guided biopsies, and discover new tools such as metagenomic sequencing.
No data sets were used in this article.
JM, AJT, and EFB conceived the project. JM formulated the first draft. All sections were edited by JM, AJT, EFB, and DBGT. The imaging section was primarily edited by FD. The final version of the paper was reviewed, edited, and approved by all authors prior to submission.
Aaron Tande and Elie Berbari report personal fees from www.UpToDate.com. Elie Berbari is also on the advisory board of Debiopharm. At least one of the (co-)authors is a member of the editorial board of Journal of Bone and Joint Infection. The peer-review process was guided by an independent editor.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was edited by Parham Sendi and reviewed by two anonymous referees.
Ahn, K.-S., Kang, C. H., Hong, S.-J., Kim, B. H., and Shim, E.: The correlation between follow-up MRI findings and laboratory results in pyogenic spondylodiscitis, BMC Musculoskel. Di., 21, 428, https://doi.org/10.1186/s12891-020-03446-4, 2020.
An, H. S. and Seldomridge, J. A.: Spinal Infections: Diagnostic Tests and Imaging Studies, Clin. Orthop., 444, 27–33, https://doi.org/10.1097/01.blo.0000203452.36522.97, 2006.
Bateman, J. L. and Pevzner, M. M.: SPINAL OSTEOMYELITIS: A REVIEW OF 10 YEARS’ EXPERIENCE, Orthopedics, 18, 561–565, https://doi.org/10.3928/0147-7447-19950601-10, 1995.
Behmanesh, B., Gessler, F., Schnoes, K., Dubinski, D., Won, S.-Y., Konczalla, J., Seifert, V., Weise, L., and Setzer, M.: Infective endocarditis in patients with pyogenic spondylodiscitis: implications for diagnosis and therapy, Neurosurg. Focus, 46, E2, https://doi.org/10.3171/2018.10.FOCUS18445, 2019.
Berbari, E. F., Kanj, S. S., Kowalski, T. J., Darouiche, R. O., Widmer, A. F., Schmitt, S. K., Hendershot, E. F., Holtom, P. D., Huddleston, P. M., Petermann, G. W., and Osmon, D. R.: 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adultsa, Clin. Infect. Dis., 61, e26–e46, https://doi.org/10.1093/cid/civ482, 2015.
Bruschwein, D. A., Brown, M. L., and McLeod, R. A.: Gallium Scintigraphy in the Evaluation of Disk-Space Infections: Concise Communication, J. Nucl. Med., 21, 925–927, 1980.
Carragee, E. J., Kim, D., van der Vlugt, T., and Vittum, D.: The Clinical Use of Erythrocyte Sedimentation Rate in Pyogenic Vertebral Osteomyelitis, Spine, 22, 2089–2093, 1997.
Carrino, J. A., Khurana, B., Ready, J. E., Silverman, S. G., and Winalski, C. S.: Magnetic Resonance Imaging-Guided Percutaneous Biopsy of Musculoskeletal Lesions, J. Bone Joint. Surg., 89, 2179–2187, https://doi.org/10.2106/JBJS.F.01230, 2007.
Chelsom, J. and Solberg, C. O.: Vertebral Osteomyelitis at a Norwegian University Hospital 1987–97: Clinical Features, Laboratory Findings and Outcome, Scand. J. Infect. Dis., 30, 147–151, https://doi.org/10.1080/003655498750003537, 1998.
Chenoweth, C. E., Bassin, B. S., Mack, M. R., Oppenlander, M. E., Patel, R. D., Quint, D. J., and Seagull, F. J.: Vertebral Osteomyelitis, Discitis, and Spinal Epidural Abscess in Adults, Quality Department guidelines report, Michigan Medicine University of Michigan, Ann Arbor (MI), available at: https://www-ncbi-nlm-nih-gov.mclibrary.idm.oclc.org/books/NBK547443/pdf/Bookshelf_NBK547443.pdf (last access: 17 January 2022), 2018.
Chew, F. S. and Kline, M. J.: Diagnostic Yield of CT-guided Percutaneous Aspiration Procedures in Suspected Spontaneous Infectious Diskitis, Radiology, 218, 211–214, https://doi.org/10.1148/radiology.218.1.r01ja06211, 2001.
Chiang, H.-Y., Chung, C.-W., Kuo, C.-C., Lo, Y.-C., Chang, W.-S., and Chi, C.-Y.: First-4-week erythrocyte sedimentation rate variability predicts erythrocyte sedimentation rate trajectories and clinical course among patients with pyogenic vertebral osteomyelitis, Plos One, 14, e0225969, https://doi.org/10.1371/journal.pone.0225969, 2019.
Choe, H., Aota, Y., Kobayashi, N., Nakamura, Y., Wakayama, Y., Inaba, Y., and Saito, T.: Rapid sensitive molecular diagnosis of pyogenic spinal infections using methicillin-resistant Staphylococcus-specific polymerase chain reaction and 16S ribosomal RNA gene-based universal polymerase chain reaction, Spine J., 14, 255–262, https://doi.org/10.1016/j.spinee.2013.10.044, 2014.
Colmenero, J. D., Ruiz-Mesa, J. D., Sanjuan-Jimenez, R., Sobrino, B., and Morata, P.: Establishing the diagnosis of tuberculous vertebral osteomyelitis, Eur. Spine J., 22, 579–586, https://doi.org/10.1007/s00586-012-2348-2, 2013.
Dagirmanjian, A., Schils, J., McHenry, M., and Modic, M. T.: MR imaging of vertebral osteomyelitis revisited., Am. J. Roentgenol., 167, 1539–1543, https://doi.org/10.2214/ajr.167.6.8956593, 1996.
De Pauw, B., Walsh, T. J., Donnelly, J. P., Stevens, D. A., Edwards, J. E., Calandra, T., Pappas, P. G., Maertens, J., Lortholary, O., Kauffman, C. A., Denning, D. W., Patterson, T. F., Maschmeyer, G., Bille, J., Dismukes, W. E., Herbrecht, R., Hope, W. W., Kibbler, C. C., Kullberg, B. J., Marr, K. A., Muñoz, P., Odds, F. C., Perfect, J. R., Restrepo, A., Ruhnke, M., Segal, B. H., Sobel, J. D., Sorrell, T. C., Viscoli, C., Wingard, J. R., Zaoutis, T., and Bennett, J. E.: Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group, Clin. Infect. Dis., 46, 1813–1821, https://doi.org/10.1086/588660, 2008.
Diehn, F. E.: Imaging of Spine Infection, Radiol. Clin. N. Am., 50, 777–798, https://doi.org/10.1016/j.rcl.2012.04.001, 2012.
Fernandez, M., Carrol, C. L., and Baker, C. J.: Discitis and Vertebral Osteomyelitis in Children: An 18-Year Review, Pediatrics, 105, 1299–1304, https://doi.org/10.1542/peds.105.6.1299, 2000.
Fuster, D., Solà, O., Soriano, A., Monegal, A., Setoain, X., Tomás, X., Garcia, S., Mensa, J., Rubello, D., and Pons, F.: A Prospective Study Comparing Whole-Body FDG PET/CT to Combined Planar Bone Scan With 67Ga SPECT/CT in the Diagnosis of Spondylodiskitis, Clin. Nucl. Med., 37, 827–832, https://doi.org/10.1097/RLU.0b013e318262ae6c, 2012.
Govender, S.: Spinal infections, J. Bone Joint Surg. Br., 87-B, 1454–1458, https://doi.org/10.1302/0301-620X.87B11.16294, 2005.
Gras, G., Buzele, R., Parienti, J. J., Debiais, F., Dinh, A., Dupon, M., Roblot, F., Mulleman, D., Marcelli, C., Michon, J., and Bernard, L.: Microbiological diagnosis of vertebral osteomyelitis: relevance of second percutaneous biopsy following initial negative biopsy and limited yield of post-biopsy blood cultures, Eur. J. Clin. Microbiol. Infect. Dis., 33, 371–375, https://doi.org/10.1007/s10096-013-1965-y, 2014.
Gratz, S., Dörner, J., Oestmann, J. W., Opitz, M., Behr, T., Meller, J., Grabbe, E., and Becker, W.: 67Ga-citrate and 99Tcm-MDP for estimating the severity of vertebral osteomyelitis, Nucl. Med. Commun., 21, 111–120, https://doi.org/10.1097/00006231-200001000-00018, 2000.
Gratz, S., Dörner, J., Fischer, U., Behr, T., Béhé, M., Altenvoerde, G., Meller, J., Grabbe, E., and Becker, W.: 18F-FDG hybrid PET in patients with suspected spondylitis, Eur. J. Nucl. Med., 29, 516–524, https://doi.org/10.1007/s00259-001-0719-8, 2002.
Gupta, A., Kowalski, T. J., Osmon, D. R., Enzler, M., Steckelberg, J. M., Huddleston, P. M., Nassr, A., Mandrekar, J. M., and Berbari, E. F.: Long-Term Outcome of Pyogenic Vertebral Osteomyelitis: A Cohort Study of 260 Patients, Open Forum Infect. Dis., 1, ofu107, https://doi.org/10.1093/ofid/ofu107, 2014.
Hadjipavlou, A. G., Cesani-Vazquez, F., Villaneuva-Meyer, J., Mader, J. T., Necessary, J. T., Crow, W., Jensen, R. E., and Chaljub, G.: The effectiveness of gallium citrate Ga 67 radionuclide imaging in vertebral osteomyelitis revisited, Am. J. Orthop. Belle Mead NJ, 27, 179–183, 1998.
Held, M., Laubscher, M., Zar, H. J., and Dunn, R. N.: GeneXpert polymerase chain reaction for spinal tuberculosis: an accurate and rapid diagnostic test, Bone Joint J., 96-B, 1366–1369, https://doi.org/10.1302/0301-620X.96B10.34048, 2014.
Heyer, C. M., Brus, L.-J., Peters, S. A., and Lemburg, S. P.: Efficacy of CT-guided biopsies of the spine in patients with spondylitis – an analysis of 164 procedures, Eur. J. Radiol., 81, e244–e249, https://doi.org/10.1016/j.ejrad.2011.02.007, 2012.
Homagk, L., Marmelstein, D., Homagk, N., and Hofmann, G. O.: SponDT (Spondylodiscitis Diagnosis and Treatment): spondylodiscitis scoring system, J. Orthop. Surg., 14, 100, https://doi.org/10.1186/s13018-019-1134-9, 2019.
Hong, S. H., Choi, J.-Y., Lee, J. W., Kim, N. R., Choi, J.-A., and Kang, H. S.: MR Imaging Assessment of the Spine: Infection or an Imitation?, RadioGraphics, 29, 599–612, https://doi.org/10.1148/rg.292085137, 2009.
Huang, Z., Zhang, Z., Yang, B., Li, W., Zhang, C., Fang, X., Zhang, C., Zhang, W., and Lin, J.: Pathogenic Detection by Metagenomic Next-Generation Sequencing in Osteoarticular Infections, Front. Cell. Infect. Mi., 10, 471, https://doi.org/10.3389/fcimb.2020.00471, 2020.
Husseini, J. S., Simeone, F. J., Nelson, S. B., and Chang, C. Y.: CT-guided discitis-osteomyelitis biopsies: needle gauge and microbiology results, Skeletal Radiol., 49, 1431–1439, https://doi.org/10.1007/s00256-020-03439-3, 2020.
Issa, K., Diebo, B. G., Faloon, M., Naziri, Q., Pourtaheri, S., Paulino, C. B., and Emami, A.: The Epidemiology of Vertebral Osteomyelitis in the United States From 1998 to 2013, Clin. Spine Surg. Spine Publ., 31, E102–E108, https://doi.org/10.1097/BSD.0000000000000597, 2018.
Iwata, E., Scarborough, M., Bowden, G., McNally, M., Tanaka, Y., and Athanasou, N. A.: The role of histology in the diagnosis of spondylodiscitis: correlation with clinical and microbiological findings, Bone Joint J., 101-B, 246–252, https://doi.org/10.1302/0301-620X.101B3.BJJ-2018-0491.R2, 2019.
Jean, M., Irisson, J.-O., Gras, G., Bouchand, F., Simo, D., Duran, C., Perronne, C., Mulleman, D., Bernard, L., and Dinh, A.: Diagnostic delay of pyogenic vertebral osteomyelitis and its associated factors, Scand. J. Rheumatol., 46, 64–68, https://doi.org/10.3109/03009742.2016.1158314, 2017.
Kamiya, N., Hatakeyama, S., Kanda, N., Yonaha, S., Akine, D., Yamamoto, Y., and Matsumura, M.: Significance of repeat magnetic resonance imaging in diagnosing vertebral osteomyelitis, J. Gen. Fam. Med., 20, 68–71, https://doi.org/10.1002/jgf2.226, 2019.
Kihira, S., Koo, C., Mahmoudi, K., Leong, T., Mei, X., Rigney, B., Aggarwal, A., and Doshi, A. H.: Combination of Imaging Features and Clinical Biomarkers Predicts Positive Pathology and Microbiology Findings Suggestive of Spondylodiscitis in Patients Undergoing Image-Guided Percutaneous Biopsy, Am. J. Neuroradiol., 41, 1316–1322, https://doi.org/10.3174/ajnr.A6623, 2020.
Kim, B. J., Lee, J. W., Kim, S. J., Lee, G. Y., and Kang, H. S.: Diagnostic Yield of Fluoroscopy-Guided Biopsy for Infectious Spondylitis, Am. J. Neuroradiol., 34, 233–238, https://doi.org/10.3174/ajnr.A3120, 2013.
Kouijzer, I. J. E., Scheper, H., de Rooy, J. W. J., Bloem, J. L., Janssen, M. J. R., van den Hoven, L., Hosman, A. J. F., Visser, L. G., Oyen, W. J. G., Bleeker-Rovers, C. P., and de Geus-Oei, L.-F.: The diagnostic value of 18F–FDG-PET/CT and MRI in suspected vertebral osteomyelitis – a prospective study, Eur. J. Nucl. Med. Mol. I., 45, 798–805, https://doi.org/10.1007/s00259-017-3912-0, 2018.
Kowalski, T. J., Layton, K. F., Berbari, E. F., Steckelberg, J. M., Huddleston, P. M., Wald, J. T., and Osmon, D. R.: Follow-Up MR Imaging in Patients with Pyogenic Spine Infections: Lack of Correlation with Clinical Features, Am. J. Neuroradiol., 28, 693–699, 2007.
Lecouvet, F., Irenge, L., Vandercam, B., Nzeusseu, A., Hamels, S., and Gala, J.-L.: The etiologic diagnosis of infectious discitis is improved by amplification-based DNA analysis, Arthritis Rheum., 50, 2985–2994, https://doi.org/10.1002/art.20462, 2004.
Ledermann, H. P., Schweitzer, M. E., Morrison, W. B., and Carrino, J. A.: MR Imaging Findings in Spinal Infections: Rules or Myths?, Radiology, 228, 506–514, https://doi.org/10.1148/radiol.2282020752, 2003.
Lefterova, M. I., Suarez, C. J., Banaei, N., and Pinsky, B. A.: Next-Generation Sequencing for Infectious Disease Diagnosis and Management, J. Mol. Diagn., 17, 623–634, https://doi.org/10.1016/j.jmoldx.2015.07.004, 2015.
Lisbona, R., Derbekyan, V., Novales-Diaz, J., and Veksler, A.: Gallium-67 Scintigraphy in Tuberculous and Nontuberculous Infectious Spondylitis, J. Nucl. Med., 34, 853–859, 1993.
Love, C., Patel, M., Lonner, B. S., Tomas, M. B., and Palestro, C. J.: Diagnosing Spinal Osteomyelitis: A Comparison of Bone and Ga-67 Scintigraphy and Magnetic Resonance Imaging, Clin. Nucl. Med., 25, 963–977, https://doi.org/10.1097/00003072-200012000-00002, 2000.
Maurer, A. H., Chen, D. C., Camargo, E. E., Wong, D. F., Wagner, H. N. J., and Alderson, P. O.: Utility of three-phase skeletal scintigraphy in suspected osteomyelitis: concise communication., J. Nucl. Med. Off. Publ. Soc. Nucl. Med., 22, 941–949, 1981.
Mavrogenis, A. F., Megaloikonomos, P. D., Igoumenou, V. G., Panagopoulos, G. N., Giannitsioti, E., Papadopoulos, A., and Papagelopoulos, P. J.: Spondylodiscitis revisited, EFORT Open Rev., 2, 447–461, https://doi.org/10.1302/2058-5241.2.160062, 2017.
McHenry, M. C., Easley, K. A., and Locker, G. A.: Vertebral Osteomyelitis: Long-Term Outcome for 253 Patients from 7 Cleveland-Area Hospitals, Clin. Infect. Dis., 34, 1342–1350, https://doi.org/10.1086/340102, 2002.
McNamara, A. L., Dickerson, E. C., Gomez-Hassan, D. M., Cinti, S. K., and Srinivasan, A.: Yield of Image-Guided Needle Biopsy for Infectious Discitis: A Systematic Review and Meta-Analysis, Am. J. Neuroradiol., 38, 2021–2027, https://doi.org/10.3174/ajnr.A5337, 2017.
Modic, M. T., Feiglin, D. H., Piraino, D. W., Boumphrey, F., Weinstein, M. A., Duchesneau, P. M., and Rehm, S.: Vertebral osteomyelitis: assessment using MR, Radiology, 157, 157–166, https://doi.org/10.1148/radiology.157.1.3875878, 1985.
Morales, H.: Infectious Spondylitis Mimics: Mechanisms of Disease and Imaging Findings, Semin, Ultrasound CT MRI, 39, 587–604, https://doi.org/10.1053/j.sult.2018.11.006, 2018.
Morcrette, H., Morgan, M. S., Farbos, A., O'Neill, P., Moore, K., Titball, R. W., and Studholme, D. J.: Genome Sequence of Staphylococcus aureus Ex1, Isolated from a Patient with Spinal Osteomyelitis, Genome Announc., 6, 1–2, https://doi.org/10.1128/genomeA.00623-18, 2018.
Mylona, E., Samarkos, M., Kakalou, E., Fanourgiakis, P., and Skoutelis, A.: Pyogenic Vertebral Osteomyelitis: A Systematic Review of Clinical Characteristics, Semin. Arthritis Rheum., 39, 10–17, https://doi.org/10.1016/j.semarthrit.2008.03.002, 2009.
Nakahara, M., Ito, M., Hattori, N., Magota, K., Takahata, M., Nagahama, K., Sudo, H., Kamishima, T., Tamaki, N., and Iwasaki, N.: 18F-FDG-PET/CT better localizes active spinal infection than MRI for successful minimally invasive surgery, Acta Radiol., 56, 829–836, https://doi.org/10.1177/0284185114541983, 2015.
Nolla, J. M., Ariza, J., Gómez-Vaquero, C., Fiter, J., Bermejo, J., Valverde, J., Escofet, D. R., and Gudiol, F.: Spontaneous pyogenic vertebral osteomyelitis in nondrug users, Semin. Arthritis Rheu., 31, 271–278, https://doi.org/10.1053/sarh.2002.29492, 2002.
Nolla-Solé, J. M., Mateo-Soria, L., Rozadilla-Sacanell, A., Mora-Salvador, J., Valverde-Garcia, J., and Roig-Escofet, D.: Role of technetium-99m diphosphonate and gallium-67 citrate bone scanning in the early diagnosis of infectious spondylodiscitis. A comparative study., Ann. Rheum. Dis., 51, 665–667, https://doi.org/10.1136/ard.51.5.665, 1992.
Osenbach, R. K., Hitchon, P. W., and Menezes, A. H.: Diagnosis and management of pyogenic vertebral osteomyelitis in adults, Surg. Neurol., 33, 266–275, https://doi.org/10.1016/0090-3019(90)90047-S, 1990.
Pandita, N., Paul, S., Yadav, G., Kalia, R. B., and Kandwal, P.: Evaluation of Challenges in Diagnosis of Spontaneous Subacute Pyogenic Spondylodiscitis in Immunocompetent Patients: Experiences from a Tertiary Care Center, Asian Spine J., 13, 621–629, https://doi.org/10.31616/asj.2018.0220, 2019.
Patel, K. B., Poplawski, M. M., Pawha, P. S., Naidich, T. P., and Tanenbaum, L. N.: Diffusion-Weighted MRI “Claw Sign” Improves Differentiation of Infectious from Degenerative Modic Type 1 Signal Changes of the Spine, Am. J. Neuroradiol., 35, 1647–1652, https://doi.org/10.3174/ajnr.A3948, 2014.
Patzakis, M. J., Rao, S., Wilkins, J., Moore, T. M., and Harvey, P. J.: Analysis of 61 cases of vertebral osteomyelitis, Clin. Orthop. Relat. Res., 264, 178–183, 1991.
Pineda, C., Espinosa, R., and Pena, A.: Radiographic Imaging in Osteomyelitis: The Role of Plain Radiography, Computed Tomography, Ultrasonography, Magnetic Resonance Imaging, and Scintigraphy, Semin. Plast. Surg., 23, 080–089, https://doi.org/10.1055/s-0029-1214160, 2009.
Prodi, E., Grassi, R., Iacobellis, F., and Cianfoni, A.: Imaging in Spondylodiskitis, Magn. Reson. Imaging C., 24, 581–600, https://doi.org/10.1016/j.mric.2016.04.005, 2016.
Pupaibool, J., Vasoo, S., Erwin, P. J., Murad, M. H., and Berbari, E. F.: The utility of image-guided percutaneous needle aspiration biopsy for the diagnosis of spontaneous vertebral osteomyelitis: a systematic review and meta-analysis, Spine J., 15, 122–131, https://doi.org/10.1016/j.spinee.2014.07.003, 2015.
Salaffi, F., Ceccarelli, L., Carotti, M., Di Carlo, M., Polonara, G., Facchini, G., Golfieri, R., and Giovagnoni, A.: Differentiation between infectious spondylodiscitis versus inflammatory or degenerative spinal changes: How can magnetic resonance imaging help the clinician?, Radiol. Med., 126, 843–859, https://doi.org/10.1007/s11547-021-01347-7, 2021.
Salipante, S. J., Sengupta, D. J., Rosenthal, C., Costa, G., Spangler, J., Sims, E. H., Jacobs, M. A., Miller, S. I., Hoogestraat, D. R., Cookson, B. T., McCoy, C., Matsen, F. A., Shendure, J., Lee, C. C., Harkins, T. T., and Hoffman, N. G.: Rapid 16S rRNA Next-Generation Sequencing of Polymicrobial Clinical Samples for Diagnosis of Complex Bacterial Infections, Plos One, 8, e65226, https://doi.org/10.1371/journal.pone.0065226, 2013.
Sheikh, A. F., Khosravi, A. D., Goodarzi, H., Nashibi, R., Teimouri, A., Motamedfar, A., Ranjbar, R., Afzalzadeh, S., Cyrus, M., and Hashemzadeh, M.: Pathogen Identification in Suspected Cases of Pyogenic Spondylodiscitis, Front. Cell. Infect. Mi., 7, 60, https://doi.org/10.3389/fcimb.2017.00060, 2017.
Smids, C., Kouijzer, I. J. E., Vos, F. J., Sprong, T., Hosman, A. J. F., de Rooy, J. W. J., Aarntzen, E. H. J. G., de Geus-Oei, L.-F., Oyen, W. J. G., and Bleeker-Rovers, C. P.: A comparison of the diagnostic value of MRI and 18F-FDG-PET/CT in suspected spondylodiscitis, Infection, 45, 41–49, https://doi.org/10.1007/s15010-016-0914-y, 2017.
Sobottke, R., Seifert, H., Fätkenheuer, G., Schmidt, M., Goßmann, A., and Eysel, P.: Current Diagnosis and Treatment of Spondylodiscitis, Dtsch. Aerzteblatt Online, 105, 181–187, https://doi.org/10.3238/arztebl.2008.0181, 2008.
Tali, E. T., Koc, A. M., and Oner, A. Y.: Spinal Brucellosis, Neuroimaging Clin. N. Am., 25, 233–245, https://doi.org/10.1016/j.nic.2015.01.004, 2015.
Tamm, A. S. and Abele, J. T.: Bone and Gallium Single-Photon Emission Computed Tomography-Computed Tomography is Equivalent to Magnetic Resonance Imaging in the Diagnosis of Infectious Spondylodiscitis: A Retrospective Study, Can. Assoc. Radiol. J., 68, 41–46, https://doi.org/10.1016/j.carj.2016.02.003, 2017.
Torda, A. J., Gottlieb, T., and Bradbury, R.: Pyogenic Vertebral Osteomyelitis: Analysis of 20 Cases and Review, Clin. Infect. Dis., 20, 320–328, https://doi.org/10.1093/clinids/20.2.320, 1995.
Waheed, G., Soliman, M. A. R., Ali, A. M., and Aly, M. H.: Spontaneous spondylodiscitis: review, incidence, management, and clinical outcome in 44 patients, Neurosurg. Focus, 46, E10, https://doi.org/10.3171/2018.10.FOCUS18463, 2019.
Wong, D., Holtom, P., and Spellberg, B.: Osteomyelitis Complicating Sacral Pressure Ulcers: Whether or Not to Treat With Antibiotic Therapy, Clin. Infect. Dis., 68, 338–342, https://doi.org/10.1093/cid/ciy559, 2019.
Wong, H., Tarr, G. P., Rajpal, K., Sweetman, L., and Doyle, A.: The impact of antibiotic pre-treatment on diagnostic yield of CT-guided biopsy for spondylodiscitis: A multi-centre retrospective study and meta-analysis, J. Med. Imaging Radiat. Oncol., 65, 146–151, https://doi.org/10.1111/1754-9485.13118, 2021.
Wu, H.-T. H., Chang, C.-Y., Chang, H., Yen, C.-C., Cheng, H., Chen, P. C.-S., and Chiou, H.-J.: Magnetic resonance imaging guided biopsy of musculoskeletal lesions, J. Chin. Med. Assoc., 75, 160–166, https://doi.org/10.1016/j.jcma.2012.02.008, 2012.
Yeh, K. J., Husseini, J. S., Hemke, R., Nelson, S. B., and Chang, C. Y.: CT-guided discitis-osteomyelitis biopsies with negative microbiology: how many days should we wait before repeating the biopsy?, Skeletal Radiol., 49, 619–623, https://doi.org/10.1007/s00256-019-03344-4, 2020.
Zarrouk, V., Feydy, A., Salles, F., Dufour, V., Guigui, P., Redondo, A., and Fantin, B.: Imaging does not predict the clinical outcome of bacterial vertebral osteomyelitis, Rheumatology, 46, 292–295, https://doi.org/10.1093/rheumatology/kel228, 2006.
Zimmerli, W.: Vertebral Osteomyelitis, N. Engl. J. Med., 362, 1022–1029, https://doi.org/10.1056/NEJMcp0910753, 2010.