the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Intravenous antibiotic duration in the treatment of prosthetic joint infection: systematic review and meta-analysis
Nour Bouji
Sijin Wen
Introduction: Long antibiotic courses, including intravenous (IV) and oral administrations, are utilized in prosthetic joint infection (PJI) treatment. This meta-analysis examines the non-inferiority of short courses (< 4 weeks) of IV antibiotics compared to long courses in treating PJI. Critical review of IV treatment is necessary due to the clinical, physical, and financial burden associated with it and its continued prolonged use in the US without much evidence to support the practice. Methods: Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), databases were searched using predefined medical subject headings (MeSH). Results: The nine included studies reported 521 total hip arthroplasties (THAs) and 530 total knee arthroplasties (TKAs). There was no significant difference in the overall success rate in short- vs. long-duration IV antibiotics for PJI treatment: odds ratio (OR) of 1.65, 95 % confidence interval (CI) of 0.78–3.46, and p=0.18. However, due to the moderate to high heterogeneity (I2=68 %, p < 0.01) amongst studies, an adjusted success rate was calculated after the exclusion of two studies. This showed a statistically significant difference between both groups (OR of 2.45, 95 % CI of 1.21–4.96, p < 0.001) favoring a short course of antibiotics and reflecting a more homogenous population (I2=51 %, p=0.06). Conclusion: This study highlights the limited data available for evaluating IV antibiotic duration in the setting of PJI. We found that a shorter duration of IV antibiotics was non-inferior to a longer duration, with an improved OR of 2.45 for treatment success, likely shortening inpatient stay as well as lessening side effects and antimicrobial resistance with a lower cost to patients and overall healthcare.
- Article
(834 KB) - Full-text XML
- BibTeX
- EndNote
Many patients with arthritis find total hip or knee replacement to be one of the most effective orthopaedic procedures for pain relief, function restoration, and improved quality of life (Zimmerli et al., 2004). As a result of the success of this surgery, the annual demand for total hip and knee arthroplasties has surpassed previously forecasted expectations. It is estimated that total hip arthroplasty (THA) and total knee arthroplasty (TKA) demand will rise by 174 % and 673 %, respectively, in the US (Sloan et al., 2018; Kurtz et al., 2007), with similar increases expected in Europe (Leitner et al., 2018; Robertsson et al., 2010). Unfortunately, prosthetic joint infection (PJI) is considered one of the leading causes of failure for hip and knee replacements and is associated with significant morbidity and mortality (Kurtz et al., 2008; Klouche et al., 2010; Bozic and Ries, 2005). According to the Nationwide Inpatient Sample, the yearly PJI incidence in the US escalated from 1.99 % to 2.18 % for hip arthroplasties and from 2.05 % to 2.18 % for knee arthroplasties from 2001 to 2009 (Kurtz et al., 2012). Despite global efforts to reduce postoperative infection, multiple international registries have also demonstrated the increased infection burden over time (Springer et al., 2017).
In general, the treatment of PJI is based on (1) surgical debridement, (2) local delivery of an antimicrobial, and (3) parenteral administration of antibiotics possibly supplemented by an oral antibiotic regimen (Tande and Patel, 2014). However, the optimal surgical and antibiotic treatment of PJI is unclear. These complicated cases place a huge physical and emotional burden on patients, challenge orthopaedic surgeons and infectious disease specialists, and strain our healthcare systems financially. In the US alone, the estimated cost of treatment of PJI is USD 1.62 billion (Haddad et al., 2017). Additionally, the increased cost to the healthcare system must be considered when it comes to the high use of resources (in hospital and outside the hospital), the length of hospital/facility stays, and the overall duration of treatment during the hospital stay (Moore et al., 2015).
There has been an increasing focus on the issue of PJI and how to improve our treatment success and decrease the burden experienced by the patient, medical professionals, and the healthcare system. Recently, the “Oral versus Intravenous Antibiotics for Bone and Joint Infection” (OVIVA) randomized, controlled non-inferiority trial was published (Li et al., 2019); this study outlined that a 1-week course of intravenous antibiotics followed by oral antibiotic step-down therapy was as effective as a longer traditional 6-week course of IV antibiotics for the management of a variety of bone or joint infections. This is one of the only comprehensive studies evaluating the topic of short vs. long treatment; however, even in this analysis, it only comprises approximately 62 % of reported cases that refer to the treatment of PJI (with the remainder being osteomyelitis and discitis).
Prior to the publication of the OVIVA trial, Yen et al. (2019) highlighted (in a systematic review and meta-analysis) that shorter clinical courses of antibiotic treatment (IV and oral) have been associated with favorable results. This review focuses on the combined course of intravenous (IV) and oral antibiotics in PJI treatment.
Currently, significant variations exist in the recommended duration of IV antibiotics alone when comparing the Infectious Diseases Society of America (IDSA) guidelines, which recommend approximately 2–6 weeks of pathogen-specific IV antibiotics (Osmon et al., 2013), with European guidelines, such as the Italian or Spanish recommendations, which tend toward a shorter IV antibiotic duration in PJI treatment (Esposito et al., 2009; Ariza et al., 2017). While most researchers focus on a shorter-duration antibiotic course, this work emphasizes the intravenous component of the antibiotic course, in the setting of PJI only, which has emerged from the requirements and the burden associated with this route of administration.
We conducted a systematic review and meta-analysis to determine if the success rate of short-duration IV antibiotics is non-inferior to a long duration of IV antibiotics. We also evaluated if baseline characteristics in the short-term group could have an impact on the success rate studied.
2.1 Search strategy
We conducted a quantitative synthesis of all studies comparing short-duration (< 4 week) and long-duration (≥ 4 weeks) IV antibiotic treatment for PJI, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines with a PRISMA checklist and algorithm (Liberati et al., 2009). The search algorithm according to the PRISMA guidelines is shown in Fig. 1. A literature review was performed using Cochrane Library, PubMed/MEDLINE, and Scopus. No restrictions were made regarding language, publication status, and clinical study design; moreover, no date restriction was set. The search strategy used the following medical subject headings (MeSH) and terms:
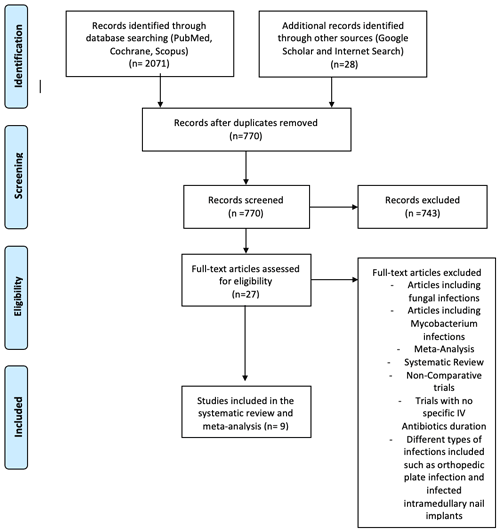
Figure 1Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the study selection process. IV denotes intravenous.
-
“intravenous antibiotic” AND “prosthetic joint infection”;
-
((“injections, intravenous” OR “infusions, intravenous”) AND (“anti-bacterial agents”)) AND (“hip prosthesis” OR “arthroplasty, replacement, hip”);
-
((“injections, intravenous” OR “infusions, intravenous”) AND (“anti-bacterial agents”)) AND (“knee prosthesis” OR “arthroplasty, replacement, knee”);
-
((“injections, intravenous” OR “infusions, intravenous”) AND (“anti-bacterial agents”)) AND (“prosthesis-related infections”).
Supplemental data were identified through a random search on Google and Google Scholar. A search of the references of recent meta-analysis on the subject was also completed. For further information regarding current trials, the ClinicalTrials.gov (ClinicalTrials.gov, 2021) registry platform was searched using the following MeSH terms: “intravenous antibiotic” AND “prosthetic joint infections”.
2.2 Data extraction
Two independent reviewers separately conducted the search. Two investigators independently screened the retrieved database for relevance, starting with title and abstract. If this was considered insufficient, full-text articles were reviewed to reach final eligibility decision. Full-text articles were reviewed according to the inclusion and exclusion criteria for the meta-analysis in order to avoid selection bias and errors. If there was any disagreement among investigators regarding the inclusion and exclusion criteria, it was solved by discussion, and the final decision was made by the senior investigator. To complete the information regarding some of the articles included in our meta-analysis, we reached out to corresponding authors via email to acquire any additional information required for the meta-analysis. When final studies to be included in the quantitative synthesis were chosen, a single author extracted and summarized data in a uniform format.
2.3 Inclusion and exclusion criteria
Studies meeting the following criteria were included: (1) human studies, (2) comparative studies, (3) reporting short-duration (< 4 weeks) vs. long-duration (≥ 4 weeks) IV antibiotic treatment in total hip or knee arthroplasty related to PJI, and (4) reporting efficacy and success rate outcomes, postoperative complications, and safety measures. Animal studies or in vitro investigations were excluded.
2.4 Quality assessment
The methodological quality of all eligible studies was independently assessed by two reviewers. The Newcastle–Ottawa scale (NOS) was used to evaluate cohort and case-control studies (Ottawa Hospital Research Institute, 2021). The NOS focuses on three areas: study group selection; group comparability; and exposure or outcome determination for case-control or cohort studies, respectively. This scale allocates a maximum of nine stars to each study, with ≥ 7 indicating a low risk of bias. Each study was rated “good”, “fair”, or “poor” based on its component scores. For this meta-analysis, a revised tool called the Risk of Bias 2 (RoB 2, 9 October 2018 version) was used to assess bias in randomized trials. The updated version of the tool is structured into five domains, including signaling questions. The five domains constitute bias related to the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, or a selection of the reported result. Each item was recorded as “high risk”, “low risk”, or “some concerns” (Sterne et al., 2019). Disagreement was resolved by a consensus strategy.
2.5 Statistical analysis
The meta-analysis and forest plots were carried out using the meta-analysis package “meta” (Schwarzer et al., 2015, 2021) in R (version 3.6.3; R Core Team). The combined odds ratio (OR) and its 95 % confidence interval (CI) was based on a random-effects model, considering between and within variation from different studies. Heterogeneity was examined based on the τ2 statistic and I2 statistic from either a random-effects model (if significant) or a fixed-effects model (if not significant) (Schwarzer et al., 2015).
The search terms identified 2071 potentially relevant studies. Other sources added 28 titles. After removing duplicates, 770 articles were screened by title and abstract, and 743 were eliminated. After applying inclusion and exclusion criteria to the remaining 27 full-text studies, 18 were excluded. The systematic review and meta-analysis included nine articles (Fig. 1).
This meta-analysis included 521 THAs and 530 TKAs on 1051 patients (Table 1). Mean age at surgery was 61–77 with a mean follow-up period of 12–75 months.
Table 1Patient demographics and study details extracted from all studies included in the meta-analysis and systematic review (n=9). Italic text denotes the treatment administered.
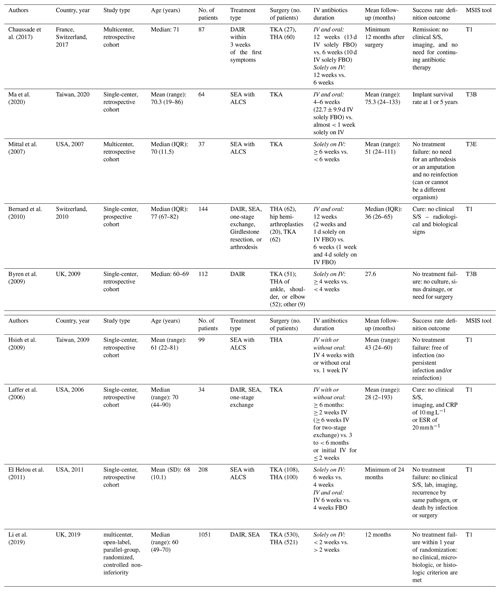
The abbreviations used in the table are as follows: IV – intravenous; S/S – signs or symptoms; ALCS – antibiotic-loaded cement spacer; DAIR – debridement, antibiotics, and implant retention; SEA – staged exchange arthroplasty; FBO – followed by oral; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; MSIS – Musculoskeletal Infection Society; IQR – interquartile range. T1 denotes Tier 1: infection control and no chronic antibiotic suppression. T3B denotes Tier 3B: septic revision > 1 year from initiation of PJI treatment. T3E denotes Tier 3E: amputation, resection, arthrodesis, and Girdlestone procedure.
To improve on uniform reporting of the definition of successful outcomes, the Musculoskeletal Infection Society (MSIS) PJI treatment outcome reporting (Fillingham et al., 2019) is highlighted in a separate column in Table 1 of this meta-analysis. This tool was used to standardize research outcome definitions and provide information that included the initial treatment of PJI. Adjustment of the components to the best-fit information provided by each article has been included, and the outcome definition is also provided in Table 1. Additionally, we evaluated the correlation to a successful outcome based on the infecting organism, such as coagulase-negative staphylococci (CoNS) or methicillin-resistant Staphylococcus aureus (MRSA); the onset of infection, with early defined as less than 3 months, delayed defined as between 3 and 12 months, and late defined as more than 12 months; the duration of infection, as described in the study; the length of hospitalization; and mortality.
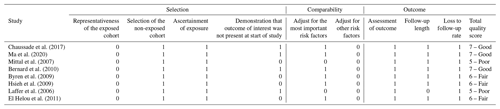
There are eight cohort studies included in the meta-analysis comparing short vs. long IV antibiotic duration in PJI treatment, but only one randomized, controlled non-inferiority trial by Li et al. (2019) was included. The focus of this analysis was on the comparison between the short and long durations of IV therapy in both groups. The risk of bias for the included cohort studies was evaluated using the “Newcastle–Ottawa Quality Assessment Form for Cohort Studies” (Ottawa Hospital Research Institute, 2021). In the quality assessment of the included studies, three were considered “good”, three were considered “fair”, and two were considered “poor” (Table 2). The risk of bias for the randomized trial (Li et al., 2019) was evaluated using the Rob 2 tool (Sterne et al., 2019) and is summarized in Table 3.
Table 2Quality assessment of the observational studies using the Newcastle–Ottawa Quality Assessment Form for Cohort Studies.
3.1 Overall success rate
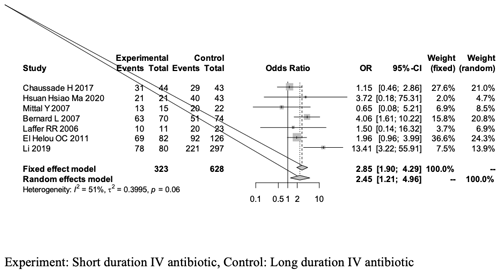
Although the measured outcomes differed across studies, we aligned the definitions of success mentioned, as some studies tracked failure rates (Table 1). Our quantitative analysis of the success rate highlighted two forest plots (see Figs. 2 and 3).
The first forest plot (Fig. 2) includes all nine studies that met the screening and inclusion criteria of our meta-analysis, and it presents the overall success rate when comparing short-duration vs. long-duration IV antibiotic treatments in THA- and TKA-related PJI treatment.
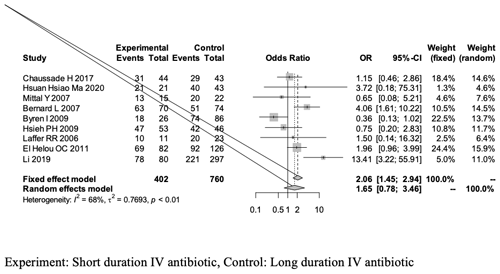
Figure 2A forest plot outlining the overall success rate of the short-duration (experimental) and long-duration (control) IV antibiotic treatments in THA- or TKA-related PJI.
The overall success rate of our meta-analysis was analyzed using a random-effects model and yielded an OR of 1.65 [0.78, 3.46]; this outcome is in favor of the experimental group (short-duration IV antibiotics), as it is greater than 1, but revealed no statistical significant difference between the two groups (95 % CI of 0.78–3.46, p = 0.18; Fig. 2, Table 4).
The overall analysis revealed a heterogeneity (estimated using I2 statistics) of 68 % (p value of < 0.01), which is considered to be between moderate and high (Higgins et al., 2003). The use of the random-effects model in the analysis is explained by the significant p value.
3.2 Adjusted success rate
As a result of the high heterogeneity in the overall success rate measured across all nine studies included in the meta-analysis, we excluded the study by Byren et al. (2009) because there was a large disparity in the number of patients between the short- and long-term groups. Hsieh et al. (2009) was excluded because both groups included were considered to be receiving short-duration IV antibiotics, which did not allow for an adequate comparison. This resulted in a second forest plot (Fig. 3) that presents an adjusted success rate outcome with a total of seven studies. The adjusted success rate after excluding the Byren et al. (2009) and Hsieh et al. (2009) studies was analyzed using a fixed-effects model and presented an OR of 2.45 [1.21, 4.96] in favor of the experimental group (short-duration IV antibiotics) with a statistically significant difference between the short- and long-duration groups favoring the shorter-duration group (95 % CI of 1.21–4.96, p < 0.001; Table 4).
Table 5Baseline variables in patients receiving short-duration (experimental) and long-duration (control) IV antibiotic treatments for PJI.
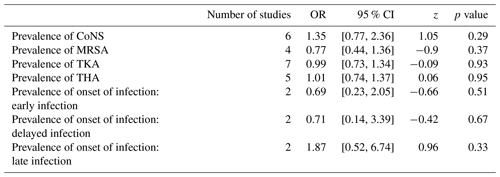
The abbreviations used in the table are as follows: CoNS – coagulase-negative staphylococci; MRSA – methicillin-resistant Staphylococcus aureus; TKA – total knee arthroplasty; THA – total hip arthroplasty.
With an I2 of 51 %, the adjusted success rate outcome analysis revealed homogeneity (p value of 0.06). Because the p value with the I2 was not significant, a fixed-effects model was used.
3.3 Baseline variables
Baseline variables among the short-duration vs. long-duration IV antibiotics groups were analyzed in our meta-analysis exploring the possibility of its influence on the endpoints of our study (Table 5). No significant difference was shown between short-duration and long-duration antibiotics when baseline variables were analyzed (Table 5). Due to the small number of studies that included them, other outcomes such as hospital length of stay (LOS) and mortality were not included in our meta-analysis.
Recently, the OVIVA trial compared oral and IV (short and long) treatment in the setting of orthopaedic infection, and a previous meta-analysis by Yen et al. (2019) compared short- and long-term antibiotic courses in PJI treatment but was not exclusive to parenteral antibiotics. To the best of our knowledge, our meta-analysis is the first and most up-to-date study comparing the short- and long-term parenteral antibiotics component for THA- and TKA-related PJI. In our meta-analysis, paper selection was different because the focus was on the IV component of treatment. In fact, some of the studies by Yen et al. (2019) were not included in our analysis because their focus was oral antibiotics; however, recent studies such as those by Ma et al. (2020) and Li et al. (2019), were added to our analysis.
With the limited studies available for review, this analysis found no significant difference in overall success rate between short- and long-duration IV antibiotics, but there was significant heterogeneity among the studies. We then chose to recalculate the success rates after the exclusion of two studies: the study by Byren et al. (2009), which compared 26 participants in the shorter-duration IV antibiotics group vs. 86 participants in the longer-duration IV antibiotics group, and the study by Hsieh et al. (2009), which compared 56 patients in the shorter-duration period vs. 51 in the longer-duration period. Once the abovementioned studies were removed, the homogeneity of the comparisons improved dramatically. With this new comparison, the adjusted success rate showed a significant difference between groups, favoring the shorter duration (OR of 2.45 [1.21, 4.96]) amongst a more homogeneous population (I2 of 51 %, p = 0.06).
Diverse studies in the literature have shown that shorter IV antibiotic treatments are more effective, although in smaller cohort studies. As described by Darley et al. (2021), a small series of infected THAs were successfully reimplanted after 14 d of IV antibiotics (range of 12–28 d) followed by 6 weeks of oral antibiotics (range of 2–25 weeks) before second-stage reimplantation, often in combination with rifampin (Darley et al., 2011). There were high success rates with a similar approach in studies by Ciriviri et al .(2015) and Ascione et al. (2017). A recent randomized pilot trial study by Manning et al. (2022) comparing short- and standard-course intravenous antibiotics for peri-prosthetic joint infections managed with debridement and implant retention showed that shorter courses of IV antibiotics may be appropriate in selected patients with early and late acute PJI managed with DAIR (debridement, antibiotics, and implant retention). Another recent study by Boclé et al. (2021), studying the effectiveness of early switching from intravenous to oral antibiotic therapy in Staphylococcus aureus prosthetic bone and joint or orthopedic metalware-associated infections, concluded that there was a low treatment failure rate in patients with S. aureus prosthetic bone and joint or orthopedic metalware-associated infection with an early oral switch from intravenous to oral antibiotic therapy.
In fact, recent evidence indicates that an early switch to oral therapy and, thus, a shorter initial duration of intravenous antibiotic treatment is effective in patients with PJIs (Boclé et al., 2021; Darley et al., 2011) and can potentially reduce the bacterial bioburden. As for the antibiotic stewardship and the risk reduction of antimicrobial resistance, as a new analysis of data from a randomized trial by Pettigrew et al. (2022) provides more evidence in support of shorter antibiotic courses for young children with non-severe community-acquired pneumonia (CAP) (Pettigrew et al., 2022); thus, this article supports the idea of “shorter is better” when it comes to antibiotic duration in the sense of less negative impact on the microbiota and less selection for resistance. Moreover, the shorter the duration of IV antibiotics, the less invasive the procedure is for patients, as IV therapy necessitates the use of an intravenous vascular access line, which can lead to infections and thromboembolic diseases (Tice et al., 2004). Long treatment courses, therefore, increase the risk of adverse events, increased length of hospital stays, influence the patient's microbiota and environment, and have a higher economic cost (Polk et al., 2004; MacDougall et al., 2005; Schindler et al., 2013; Valour et al., 2014).
Comparing baseline variables like infection prevalence and acuity between groups showed no significant differences. Baseline variables also included differences in surgical treatments; DAIR and a staged exchange arthroplasty were emphasized in most studies, but this was limited by study numbers. The IDSA (Osmon et al., 2013) recommends 2–6 weeks of pathogen-specific IV antibiotics after DAIR and one-stage exchange. Antibiotics are generally prescribed for shorter durations in European guidelines: a 15 d course of IV antibiotics is recommended in clinical practice by the Société de Pathologie Infectieuse de Langue Française for bone and joint prosthetic device infection (Société de Pathologie Infectieuse de Langue Française (SPILF) et al., 2010), and Italian guidelines recommend that, after resection arthroplasty, 2–3 weeks of intravenous antibiotics be administered. As a result, of our meta-analysis, most of the short-duration IV antibiotic components of the treatment lasted less than 2 weeks, favoring a shorter-duration effect.
Our study is limited due to the inherent limitations of a meta-analyses. The validity is dependent on the quality and quantity of studies that are used. Our meta-analysis included eight cohort studies and only one randomized clinical trial. However, the eight cohort studies included have a Newcastle–Ottawa scale rating of 5 or higher, indicating that the study is of intermediate or higher quality. A further limitation of this meta-analysis is the difficulty in interpreting the conclusive results, as (although the duration of IV antibiotics was evaluated) some studies focused solely on the IV component, whereas others reflected clinical practice by showing intravenous antibiotics followed by oral antibiotic therapy. In addition, we were not able to adhere to heterogeneity in all aspects of study design, such as differences in surgical treatments, the pathogens identified, and the selection of antimicrobials. More homogeneity is needed in future research. Studies from a wide range of environments and from different countries including patients of various races may provide generalizability that could be beneficial. Furthermore, there was no consistent definition of success rate across studies: some reported remission rates, whereas others reported failure rates, which we adjusted to a success rate definition along with the number reported in the study. In addition, the MSIS classification as per outcome reported was adjusted to the best fit based on the information provided by each study.
There is evidence that short-duration IV antibiotic treatment is not inferior to long-duration IV antibiotics and that the impact of transitioning to a short course of treatment could be significant in improving the care provided to patients suffering from PJI. However, there is a paucity of literature to support this change in practice. To ensure that this transition is made safely, further evaluation should occur. Given the rarity of this complication, it would likely require a multicenter, randomized, controlled trial.
The additional information used in this work can be accessed by contacting Matthew Scarborough (matthew.scarborough@ouh.nhs.uk) or Lilian Mwango, the data curator (lmwango@kemri-wellcome.org) for the OVIVA Trial.
MJD conceived of the presented idea. NB developed the theory and performed the computations. NB and SW verified the analytical methods and results. MJD supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
The contact author has declared that none of the authors has any competing interests.
This study is a meta-analysis; therefore, the authors were not required to obtain any written informed consent from patients nor the approval of the institutional review board before submitting the publication.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was edited by Bryan Springer and reviewed by two anonymous referees.
Ariza, J., Cobo, J., Baraia-Etxaburu, J., Benito, N., Bori, G., Cabo, J., Corona, P., Esteban, J., Horcajada, J. P., Lora-Tamayo, J., Murillo, O., Palomino, J., Parra, J., Pigrau, C., Del Pozo, J. L., Riera, M., Rodríguez, D., Sánchez-Somolinos, M., Soriano, A., Del Toro, M. D., de la Torre, B., Spanish Network for the Study of Infectious Diseases and the Sociedad Española de Enfermedades Infecciosas, and Microbiología Clínica (SEIMC): Executive summary of management of prosthetic joint infections, Clinical practice guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Enferm. Infecc. Microbiol. Clin., 35, 189–195, https://doi.org/10.1016/j.eimc.2016.08.012, 2017.
Ascione, T., Pagliano, P., Balato, G., Mariconda, M., Rotondo, R., and Esposito, S.: Oral Therapy, Microbiological Findings, and Comorbidity Influence the Outcome of Prosthetic Joint Infections Undergoing 2-Stage Exchange, J. Arthroplasty, 32, 2239–2243, https://doi.org/10.1016/j.arth.2017.02.057, 2017.
Bernard, L., Legout, L., Zürcher-Pfund, L., Stern, R., Rohner, P., Peter, R., Assal, M., Lew, D., Hoffmeyer, P., and Uçkay, I.: Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty, J. Infect., 61, 125–132, https://doi.org/10.1016/j.jinf.2010.05.005, 2010.
Boclé, H., Lavigne, J.-P., Cellier, N., Crouzet, J., Kouyoumdjian, P., Sotto, A., and Loubet, P.: Effectiveness of early switching from intravenous to oral antibiotic therapy in Staphylococcus aureus prosthetic bone and joint or orthopedic metalware-associated infections, BMC Musculoskelet. Disord., 22, 1–7, 2021.
Bozic, K. J. and Ries, M. D.: The Impact of Infection After Total Hip Arthroplasty on Hospital and Surgeon Resource Utilization, J. Bone Joint Surg. Am., 87, 1746–1751, https://doi.org/10.2106/JBJS.D.02937, 2005.
Byren, I., Bejon, P., Atkins, B. L., Angus, B., Masters, S., McLardy-Smith, P., Gundle, R., and Berendt, A.: One hundred and twelve infected arthroplasties treated with “DAIR” (debridement, antibiotics and implant retention): antibiotic duration and outcome, J. Antimicrob. Chemother., 63, 1264–1271, https://doi.org/10.1093/jac/dkp107, 2009.
Chaussade, H., Uçkay, I., Vuagnat, A., Druon, J., Gras, G., Rosset, P., Lipsky, B. A., and Bernard, L.: Antibiotic therapy duration for prosthetic joint infections treated by Debridement and Implant Retention (DAIR): Similar long-term remission for 6 weeks as compared to 12 weeks, Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis., 63, 37–42, https://doi.org/10.1016/j.ijid.2017.08.002, 2017.
Ciriviri, J., Talevski, D., Nestorovski, Z., Vraniskoski, T., and Mishevska-Perchinkova, S.: A Two Phase Treatment of an Infected Hip Endoprosthesis, Pril. Makedon. Akad. Na Nauk. Umet. Oddelenie Za Med. Nauki, 36, 195–202, https://doi.org/10.1515/prilozi-2015-0067, 2015.
ClinicalTrials.gov: ClinicalTrials.gov, https://clinicaltrials.gov/, last access: 12 September 2021.
Darley, E. S. R., Bannister, G. C., Blom, A. W., Macgowan, A. P., Jacobson, S. K., and Alfouzan, W.: Role of early intravenous to oral antibiotic switch therapy in the management of prosthetic hip infection treated with one- or two-stage replacement, J. Antimicrob. Chemother., 66, 2405–2408, https://doi.org/10.1093/jac/dkr277, 2011.
El Helou, O. C., Berbari, E. F., Lahr, B. D., Marculescu, C. E., Razonable, R. R., Steckelberg, J. M., Hanssen, A. D., and Osmon, D. R.: Management of prosthetic joint infection treated with two-stage exchange: the impact of antimicrobial therapy duration, Curr. Orthop. Pract., 22, 333–338, https://doi.org/10.1097/BCO.0b013e318221813a, 2011.
Esposito, S., Leone, S., Bassetti, M., Borrè, S., Leoncini, F., Meani, E., Venditti, M., Mazzotta, F., and Bone Joint Infections Committee for the Italian Society of Infectious Tropical Diseases (SIMIT): Italian guidelines for the diagnosis and infectious disease management of osteomyelitis and prosthetic joint infections in adults, Infection, 37, 478–496, https://doi.org/10.1007/s15010-009-8269-2, 2009.
Fillingham, Y. A., Della Valle, C. J., Suleiman, L. I., Springer, B. D., Gehrke, T., Bini, S. A., Segreti, J., Chen, A. F., Goswami, K., Tan, T. L., Shohat, N., Diaz-Ledezma, C., Schwartz, A. J., and Parvizi, J.: Definition of Successful Infection Management and Guidelines for Reporting of Outcomes After Surgical Treatment of Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society (MSIS), J. Bone Joint Surg. Am., 101, e69, https://doi.org/10.2106/JBJS.19.00062, 2019.
Haddad, F. S., Ngu, A., and Negus, J. J.: Prosthetic Joint Infections and Cost Analysis?, in: A Modern Approach to Biofilm-Related Orthopaedic Implant Infections: Advances in Microbiology, Infectious Diseases and Public Health Volume 5, edited by: Drago, L., Springer International Publishing, Cham, 93–100, https://doi.org/10.1007/5584_2016_155, 2017.
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G.: Measuring inconsistency in meta-analyses, BMJ, 327, 557–560, https://doi.org/10.1136/bmj.327.7414.557, 2003.
Hsieh, P.-H., Huang, K.-C., Lee, P.-C., and Lee, M. S.: Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy, J. Antimicrob. Chemother., 64, 392–397, https://doi.org/10.1093/jac/dkp177, 2009.
Klouche, S., Sariali, E., and Mamoudy, P.: Total hip arthroplasty revision due to infection: A cost analysis approach, Orthop. Traumatol. Surg. Res., 96, 124–132, https://doi.org/10.1016/j.otsr.2009.11.004, 2010.
Kurtz, S., Ong, K., Lau, E., Mowat, F., and Halpern, M.: Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030, J. Bone Joint Surg. Am., 89, 780–785, https://doi.org/10.2106/JBJS.F.00222, 2007.
Kurtz, S. M., Lau, E., Schmier, J., Ong, K. L., Zhao, K., and Parvizi, J.: Infection Burden for Hip and Knee Arthroplasty in the United States, J. Arthroplasty, 23, 984–991, https://doi.org/10.1016/j.arth.2007.10.017, 2008.
Kurtz, S. M., Lau, E., Watson, H., Schmier, J. K., and Parvizi, J.: Economic Burden of Periprosthetic Joint Infection in the United States, J. Arthroplasty, 27, 61–65.e1, https://doi.org/10.1016/j.arth.2012.02.022, 2012.
Laffer, R. R., Graber, P., Ochsner, P. E., and Zimmerli, W.: Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre, Clin. Microbiol. Infect., 12, 433–439, https://doi.org/10.1111/j.1469-0691.2006.01378.x, 2006.
Leitner, L., Türk, S., Heidinger, M., Stöckl, B., Posch, F., Maurer-Ertl, W., Leithner, A., and Sadoghi, P.: Trends and Economic Impact of Hip and Knee Arthroplasty in Central Europe: Findings from the Austrian National Database, Sci. Rep., 8, 4707, https://doi.org/10.1038/s41598-018-23266-w, 2018.
Li, H.-K., Rombach, I., Zambellas, R., Walker, A. S., McNally, M. A., Atkins, B. L., Lipsky, B. A., Hughes, H. C., Bose, D., Kümin, M., Scarborough, C., Matthews, P. C., Brent, A. J., Lomas, J., Gundle, R., Rogers, M., Taylor, A., Angus, B., Byren, I., Berendt, A. R., Warren, S., Fitzgerald, F. E., Mack, D. J. F., Hopkins, S., Folb, J., Reynolds, H. E., Moore, E., Marshall, J., Jenkins, N., Moran, C. E., Woodhouse, A. F., Stafford, S., Seaton, R. A., Vallance, C., Hemsley, C. J., Bisnauthsing, K., Sandoe, J. A. T., Aggarwal, I., Ellis, S. C., Bunn, D. J., Sutherland, R. K., Barlow, G., Cooper, C., Geue, C., McMeekin, N., Briggs, A. H., Sendi, P., Khatamzas, E., Wangrangsimakul, T., Wong, T. H. N., Barrett, L. K., Alvand, A., Old, C. F., Bostock, J., Paul, J., Cooke, G., Thwaites, G. E., Bejon, P., and Scarborough, M.: Oral versus Intravenous Antibiotics for Bone and Joint Infection, N. Engl. J. Med., 380, 425–436, https://doi.org/10.1056/NEJMoa1710926, 2019.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., Clarke, M., Devereaux, P. J., Kleijnen, J., and Moher, D.: ThePRISMA statement for reporting systematic reviews and meta-analyses ofstudies that evaluate healthcare interventions: explanation and elaboration, BMJ, 339, b2700, https://doi.org/10.1136/bmj.b2700, 2009.
Ma, H.-H., Chou, T.-F. A., Tsai, S.-W., Chen, C.-F., Wu, P.-K., Chen, C.-M., and Chen, W.-M.: Is short-course systemic antibiotic therapy using an antibiotic-loaded cement spacer safe after resection for infected total knee arthroplasty? A comparative study, J. Formos. Med. Assoc. Taiwan Yi Zhi, 119, 1070–1079, https://doi.org/10.1016/j.jfma.2019.10.001, 2020.
MacDougall, C., Powell, J. P., Johnson, C. K., Edmond, M. B., and Polk, R. E.: Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., 41, 435–440, https://doi.org/10.1086/432056, 2005.
Manning, L., Metcalf, S., Dymock, M., Robinson, O., Clark, B., Nelson, R., Paterson, D. L., Yates, P., Loewenthal, M., and Dewar, D.: Short-versus standard-course intravenous antibiotics for peri-prosthetic joint infections managed with debridement and implant retention: a randomised pilot trial using a desirability of outcome ranking (DOOR) endpoint, Int. J. Antimicrob. Agents, 60, 106598, https://doi.org/10.1016/j.ijantimicag.2022.106598, 2022.
Mittal, Y., Fehring, T. K., Hanssen, A., Marculescu, C., Odum, S. M., and Osmon, D.: Two-stage reimplantation for periprosthetic knee infection involving resistant organisms, J. Bone Joint Surg. Am., 89, 1227–1231, https://doi.org/10.2106/JBJS.E.01192, 2007.
Moore, A. J., Blom, A. W., Whitehouse, M. R., and Gooberman-Hill, R.: Deep prosthetic joint infection: a qualitative study of the impact on patients and their experiences of revision surgery, BMJ Open, 5, e009495, https://doi.org/10.1136/bmjopen-2015-009495, 2015.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., Wilson, W. R., and Infectious Diseases Society of America: Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., 56, e1–e25, https://doi.org/10.1093/cid/cis803, 2013.
Ottawa Hospital Research Institute: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, last access: 12 September 2021.
Pettigrew, M. M., Kwon, J., Gent, J. F., Kong, Y., Wade, M., Williams, D. J., Creech, C. B., Evans, S., Pan, Q., Walter, E. B., and Martin, J. M.: Comparison of the Respiratory Resistomes and Microbiota in Children Receiving Short versus Standard Course Treatment for Community-Acquired Pneumonia, Mbio, 13, e00195-22, https://doi.org/10.1128/mbio.00195-22, 2022.
Polk, R. E., Johnson, C. K., McClish, D., Wenzel, R. P., and Edmond, M. B.: Predicting hospital rates of fluoroquinolone-resistant Pseudomonas aeruginosa from fluoroquinolone use in US hospitals and their surrounding communities, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., 39, 497–503, https://doi.org/10.1086/422647, 2004.
Robertsson, O., Bizjajeva, S., Fenstad, A. M., Furnes, O., Lidgren, L., Mehnert, F., Odgaard, A., Pedersen, A. B., and Havelin, L. I.: Knee arthroplasty in Denmark, Norway and Sweden, Acta Orthop., 81, 82–89, https://doi.org/10.3109/17453671003685442, 2010.
Schindler, M., Bernard, L., Belaieff, W., Gamulin, A., Racloz, G., Emonet, S., Lew, D., Hoffmeyer, P., and Uçkay, I.: Epidemiology of adverse events and Clostridium difficile-associated diarrhea during long-term antibiotic therapy for osteoarticular infections, J. Infect., 67, 433–438, https://doi.org/10.1016/j.jinf.2013.07.017, 2013.
Schwarzer, G., Carpenter, J. R., and Rücker, G.: Meta-Analysis with R, Springer International Publishing, https://doi.org/10.1007/978-3-319-21416-0, 2015.
Schwarzer, G., Carpenter, J. R., and Rücker, G.: Auth. Meta-Analysis With R | PDF | R (Programming Language) | Command Line Interface, https://www.scribd.com/document/394070235/Guido-Schwarzer-James-R-Carpenter-Gerta-Rucker-Auth-Meta-Analysis-With-R, last access: 12 September 2021.
Sloan, M., Premkumar, A., and Sheth, N. P.: Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030, J. Bone Joint Surg. Am., 100, 1455–1460, https://doi.org/10.2106/JBJS.17.01617, 2018.
Société de Pathologie Infectieuse de Langue Française (SPILF), Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT), Groupe de Pathologie Infectieuse Pédiatrique (GPIP), Société Française d'Anesthésie et de Réanimation (SFAR), Société Française de Chirurgie Orthopédique et Traumatologique (SOFCOT), Société Française d'Hygiène Hospitalière (SFHH), Société Française de Médecine Nucléaire (SFMN), Société Française de Médecine Physique et de Réadaptation (SOFMER), Société Française de Microbiologie (SFM), Société Française de Radiologie (SFR-Rad), and Société Française de Rhumatologie (SFR-Rhu): Recommendations for bone and joint prosthetic device infections in clinical practice (prosthesis, implants, osteosynthesis), Société de Pathologie Infectieuse de Langue Française, Med. Mal. Infect., 40, 185–211, https://doi.org/10.1016/j.medmal.2009.12.009, 2010.
Springer, B. D., Cahue, S., Etkin, C. D., Lewallen, D. G., and McGrory, B. J.: Infection burden in total hip and knee arthroplasties: an international registry-based perspective, Arthroplasty Today, 3, 137–140, 2017.
Sterne, J. A., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., Cates, C. J., Cheng, H.-Y., Corbett, M. S., and Eldridge, S. M.: RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ, 366, l4898, https://doi.org/10.1136/bmj.l4898, 2019.
Tande, A. J. and Patel, R.: Prosthetic Joint Infection, Clin. Microbiol. Rev., 27, 302–345, https://doi.org/10.1128/CMR.00111-13, 2014.
Tice, A. D., Rehm, S. J., Dalovisio, J. R., Bradley, J. S., Martinelli, L. P., Graham, D. R., Gainer, R. B., Kunkel, M. J., Yancey, R. W., Williams, D. N., and IDSA: Practice guidelines for outpatient parenteral antimicrobial therapy, IDSA guidelines, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., 38, 1651–1672, https://doi.org/10.1086/420939, 2004.
Valour, F., Karsenty, J., Bouaziz, A., Ader, F., Tod, M., Lustig, S., Laurent, F., Ecochard, R., Chidiac, C., and Ferry, T.: Antimicrobial-Related Severe Adverse Events during Treatment of Bone and Joint Infection Due to Methicillin-Susceptible Staphylococcus aureus, Antimicrob. Agents Chemother., 58, 746–755, https://doi.org/10.1128/AAC.02032-13, 2014.
Yen, H.-T., Hsieh, R. W., Huang, C., Hsu, T.-C., Yeh, T., Chen, Y.-C., Chen, W.-S., and Lee, C.-C.: Short-course versus long-course antibiotics in prosthetic joint infections: a systematic review and meta-analysis of one randomized controlled trial plus nine observational studies, J. Antimicrob. Chemother., 74, 2507–2516, https://doi.org/10.1093/jac/dkz166, 2019.
Zimmerli, W., Trampuz, A., and Ochsner, P. E.: Prosthetic-Joint Infections, N. Engl. J. Med., 351, 1645–1654, https://doi.org/10.1056/NEJMra040181, 2004.