the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The proportion of chronic periprosthetic joint infection patients with Candida isolates
Samuelson E. Osifo
Adrian Santana
Michael F. Shannon
Victoria R. Wong
Caroline F. Tyndall
Christian Cisneros
Niosha Parvizi
Brian A. Klatt
Johannes F. Plate
Nicolas S. Piuzzi
Introduction: Fungal periprosthetic joint infection (PJI) has historically been reported in 1 %–2 % of cases, with Candida species accounting for most isolates. However, the true incidence is likely underestimated. Standard aerobic and anaerobic culture techniques have limited sensitivity for detecting fungi, single positive fungal cultures are often excluded or inconsistently classified, culture-negative infections may mask low-burden fungal pathogens, and polymicrobial cultures may obscure the contribution of fungal organisms. The objective of this study was to quantify the burden of potentially unrecognized fungal involvement and provide a more accurate estimate of the incidence of Candida-associated PJI. Methods: Following a systematic literature search, we performed a quantitative sensitivity analysis using imputation with informative missingness odds ratios (IMORs). Reported Candida cases were adjusted for four predefined sources of under-ascertainment: single positive cultures, negative cultures, polymicrobial cultures, and variability in fungal culture sensitivity. Results: 23 studies met inclusion criteria, reporting a total of 28 253 PJI patients, of whom 590 had Candida involvement (2.1 %; range 0.9 %–10.1 %). After imputation for missing data, the estimated proportion of PJI cases involving Candida ranged from 1.4 %–13.6 %, with a mean of 5.1 %. The odds ratios for known risk factors for chronic refractory PJI exceeded 2.0, suggesting the proportion of Candida in this population likely exceeds 10 %. Conclusion: The involvement of Candida in PJI is likely underreported. The adjusted incidence is approximately 5 % across all PJI cases. Among patients with chronic refractory PJI, especially those that have failed multiple surgeries, the incidence of Candida PJI is approximately 10 %. Level of Evidence: Level III.
- Article
(729 KB) - Full-text XML
- BibTeX
- EndNote
Periprosthetic joint infection (PJI) remains the most serious complication of hip and knee arthroplasty globally and the leading cause of early revision in total knee arthroplasty (Del Pozo and Patel, 2009; Kurtz et al., 2007). Fungal PJI has traditionally been considered rare, historically reported in 1 %–2 % of PJI cases (Sambri et al., 2022; Sidhu et al., 2019; Tande and Patel, 2014). However, contemporary evidence from large primary cohorts suggests that fungal organisms may be present in a substantially higher proportion (up to 10 %) of PJI cases (Brown et al., 2018; Cao et al., 2025). Candida species account for approximately 95 % of culture-confirmed fungal PJIs (Benito et al., 2016; Koutserimpas et al., 2022; Herndon et al., 2023; McCulloch et al., 2023).
Several factors likely contribute to systematic underestimation of fungal involvement in chronic PJI. Conventional aerobic and anaerobic culture methods have reduced sensitivity for Candida, particularly in low-burden infections or polymicrobial communities, making both single isolates and mixed fungal–bacterial infections difficult to detect (Watanabe et al., 2024). Underreporting may also occur in culture-negative cases, especially in institutions without optimized fungal culture protocols or prolonged incubation. Diagnostic uncertainty is further compounded when a single Candida isolate is dismissed as contamination, leading to misclassification. Additionally, many published series further aggregate acute and chronic PJI, although fungal infections occur predominantly in chronic refractory cases.
The objective of this study was to assess how these diagnostic and reporting limitations influenced the observed incidence of fungal PJI. Specifically, we aimed to (1) establish the baseline proportion of Candida isolates among confirmed PJI cases; (2) identify the frequency of missing, excluded, or misclassified variables that could obscure true fungal involvement; and (3) quantify the adjusted incidence of Candida-associated PJI after correcting for potential underreporting.
2.1 Study design
This study was a quantitative sensitivity analysis of published clinical series reporting microbiologic findings in confirmed periprosthetic joint infection (PJI). The primary outcome was the proportion of PJI cases with evidence of Candida involvement. The analysis was designed to evaluate the impact of incomplete or inconsistently reported microbiologic data on observed estimates of Candida involvement across heterogeneous clinical studies.
2.2 Data sources and study selection
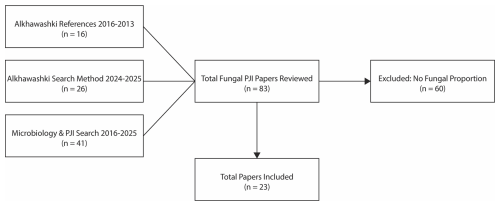
A recent meta-analysis identified English-language publications reporting outcomes on ≥5 patients with diagnosed hip or knee fungal PJI between 2009 and 2023 (Alkhawashki et al., 2025). All studies published between 2016 and 2023 included in this meta-analysis were reviewed. To extend capture beyond this cohort, a structured literature search was independently conducted for studies published from January 2024 through October 2025 using PubMed. The primary search string was “fungal periprosthetic joint infection”, applied to titles and abstracts. In parallel, a secondary PubMed search was performed, following the methodology described by Tai et al. (2022a), using the Boolean search strategy “hip” OR “knee” AND “periprosthetic joint infection” AND “microbiology aetiology” for publications from 2016 through October 2025. Reviewed studies were included if they reported microbiologic results from preoperative or intraoperative samples and allowed calculation of the proportion of PJI cases with evidence of Candida involvement. Studies were excluded if microbiologic data were insufficient to determine Candida involvement. This process yielded 23 studies for inclusion (Fig. 1).
2.3 Identification of missing microbiologic data
The proportion of PJI patients with Candida-positive preoperative or intraoperative cultures was calculated for each study included. Review of included studies revealed inconsistent methodology and reporting practices, resulting in missing diagnostic data relevant to the calculated proportion of Candida involvement. Four prespecified sources of missingness were identified:
-
Culture-negative PJI cases included without clarification of fungal assessment;
-
Polymicrobial PJI cases without species-level reporting, where fungal organisms may have been present but not specified;
-
Exclusion of single positive Candida isolates, commonly dismissed as contaminants;
-
Variable sensitivity of microbiologic methods, with potential under-detection of fungal organisms.
2.4 Sensitivity analysis and imputation strategy
A quantitative sensitivity analysis was conducted using imputation with informative missingness odds ratios (IMORs) for each missingness category, following methods for imputing missing outcome data in meta-analyses of clinical trials (Higgins et al., 2008). For each study, the expected number of missed Candida cases was estimated under both best-case and worst-case scenarios. These expected cases were added to the reported number of Candida infections, and minimum and maximum adjusted proportions were calculated. A sample size-weighted mean and range of expected Candida proportions was generated across all included studies.
Consistent with Higgins et al. (2008), the analysis did not assume that missing patients were either entirely “all Candida” or “no Candida.” Instead, each category of missingness was assigned a relative risk (RR) of Candida involvement based on published clinical data. Applying these literature-derived probabilities provides more realistic estimates and reduces the standard error compared with traditional all-or-none assumptions. The additional number of expected Candida cases for each missingness category was calculated as , where mi represents the number of PJI cases affected by that specific source of missingness.
The number of PJI patients with missing data for Candida is often reported directly in the paper (e.g., number of culture-negative patients). If such patients are excluded, or not reported and assumed to be excluded in worst-case sensitivity calculations, the number of missing patients (mi) was calculated by multiplying the total number of PJI patients in the study (Ni) by the proportion (pi) of PJI patients in the literature with the same reason for missingness (e.g., percentage of culture-negative PJI cases): .
The relative risk of Candida for each missingness category (RRi) was derived from the literature. For missed cases attributable to insensitive microbiologic test methods, RR was calculated as the difference in the probability of detecting Candida using high-sensitivity methods compared with conventional culture techniques reported in the publication. Imputation was restricted to Candida involvement because fungal organisms are inconsistently assessed and variably reported across published PJI series, with frequent exclusion of single positive cultures, lack of species-level reporting in polymicrobial infections, and heterogeneous use of high-sensitivity diagnostic methods. These factors introduce systematic uncertainty in estimates of fungal involvement that cannot be addressed by standard reporting alone.
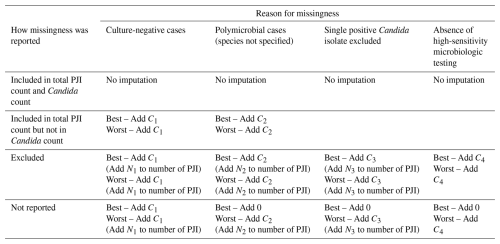
2.5 Best-case and worst-case scenarios
Best-case and worst-case scenarios were defined to bound the plausible range of Candida involvement under varying assumptions regarding missing microbiologic data. The best-case and worst-case scenarios of the sensitivity analysis for each missingness reason were determined (Table 1). These scenarios depend on whether PJI patients were included in the study without microbial data, specifically excluded from the study, or not reported (and therefore potentially included or excluded). When patients with expected Candida involvement were known to be missing, they were added to both the best-case and worst-case scenarios. If the number of PJI patients affected by a missingness category was not reported, no imputation was performed for the best-case scenario (Add 0). When PJI patients were excluded or assumed to be excluded in worst-case scenarios, the number of missing PJI patients for that reason (Ni) was added to the total number of PJI patients before recalculating the proportion of patients expected to have Candida involvement, to avoid overestimation.
2.6 Informative missingness odds ratios (IMORs)
The relative risks associated with each missingness category were derived from published literature reporting the proportion of cases with Candida isolates associated with each source of missingness, including the following:
-
Detection of Candida in culture-negative PJI using next-generation sequencing (NGS).
-
The proportion of Candida in polymicrobial PJI.
-
The frequency of single positive Candida cultures in PJI.
-
The detection of Candida by high-sensitivity microbial assays missed by conventional cultures.
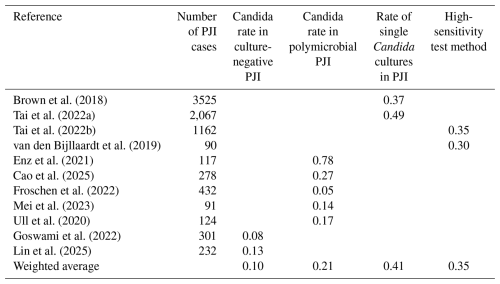
The IMOR for each category was calculated as a weighted average across included studies (Table 2).
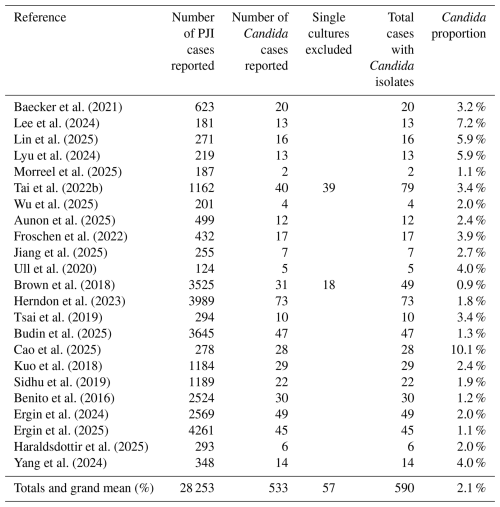
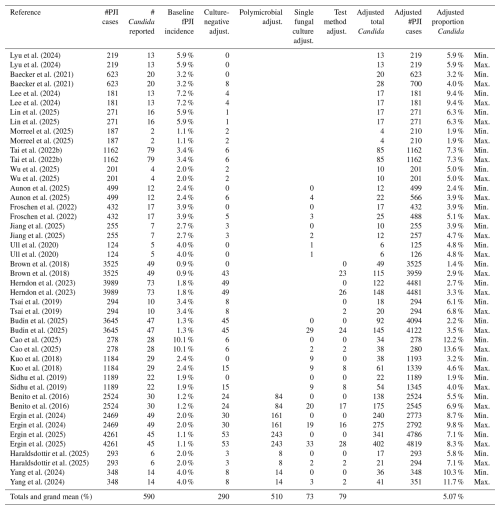
A total of 23 studies met inclusion criteria, reporting a combined total of 28 253 PJI patients (Table 3). Candida PJI was identified in 533 patients (1.9 %). When patients with a single positive Candida culture were included, the total increased to 590 patients (2.1 %). Across all included studies, the reported incidence of Candida PJI ranged from 0.9 % to 10.1 %.
Each study was assessed for missing diagnostic data that could lead to underestimation of Candida involvement (Table 4). The major sources of missingness included (1) culture-negative PJI cases in which fungal involvement could not be excluded, (2) polymicrobial PJI cases where the full microbial profile was not reported, (3) exclusion or non-classification of single positive Candida isolates, and (4) the absence of high-sensitivity microbiologic testing such as fungal cultures, next-generation sequencing (NGS), or sonication.
Table 4Summary of missing data categories requiring imputation for each study.
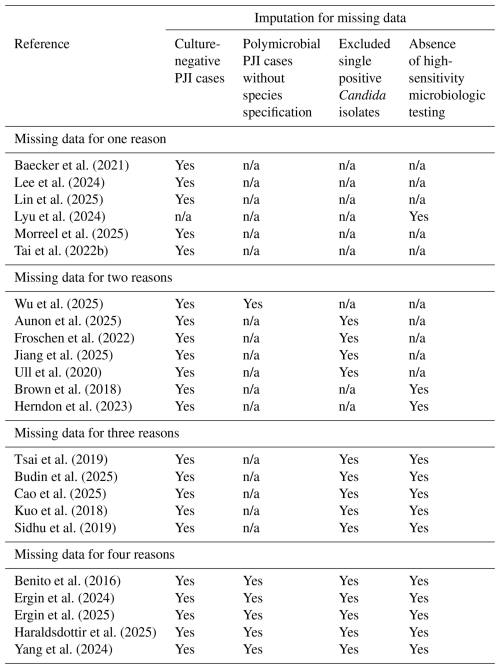
n/a = not applicable; no imputation required for that category.
Among all included studies, only the series by Lyu et al. (2024), which used NGS to detect Candida in culture-negative cases, did not require imputation for the culture-negative category. Missingness due to unreported polymicrobial species required imputation in 6 studies, exclusion of single positive Candida isolates in 13 studies, and absence of high-sensitivity culture methods in 12 studies. The incidence of Candida PJI was then recalculated after applying imputation for each missingness category (Table 5).
Table 5Sensitivity analysis: minimum and maximum expected proportion of Candida in confirmed PJI cases.
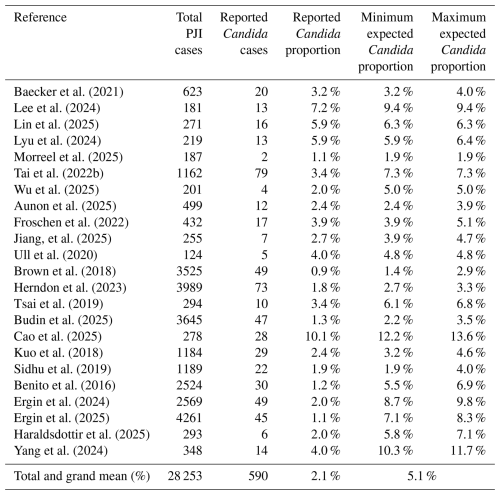
PJI – periprosthetic joint infection.
After adjusting for missing data, the expected proportion of Candida PJI increased to 5.1 % across all studies (range: 1.4 %–13.6 %). This estimate was derived by adding the expected number of additional Candida cases for each missingness variable (Table A1). Most adjustments were attributable to unreported microbial species in polymicrobial cases (53 %) and unknown microbiology in culture-negative PJI cases (30 %).
As a sensitivity check, the subset of six studies requiring only one imputation correction, representing the lowest potential risk of error from multiple imputations, yielded an expected Candida incidence of 5.9 % (range: 1.9 % to 9.4 %). This is consistent with the overall adjusted estimate and supports the robustness of the findings.
This quantitative sensitivity analysis suggests that the contribution of Candida to PJI is substantially underestimated in the published literature. After imputing missing microbiologic data across 28 253 confirmed PJI cases from 23 clinical studies, the expected proportion of Candida-associated PJI increased to approximately 5 %–6 % (range: 1.4 %–13.6 %). This estimate is more than double the conventionally reported incidence of 1 %–2 % and represents a clinically meaningful difference for diagnosis, treatment planning, and prognostication. By explicitly accounting for multiple, well-described sources of microbiologic under-ascertainment, this analysis provides an adjusted estimate of Candida involvement that complements existing epidemiologic reports. Notably, more than half of the included studies were published within the past decade, with a marked increase in reports from 2024–2025, reflecting growing recognition of fungal involvement in PJI.
Missing data were common and required imputation for all of the 23 studies. The most frequent source of missingness was failure to report organism-level microbiology in polymicrobial infections. Because polymicrobial PJI appears to carry a relatively high expected proportion of Candida, greater than 20 % in this analysis, failure to characterize all identified organisms may substantially underestimate fungal involvement.
Although the expected proportion of Candida was calculated for all reported PJI cases, fungal pathogens are most frequently encountered in chronic refractory infections and in sub-populations with risk factors associated with chronic PJI treatment failure. In a recent series of 193 chronic PJI cases treated with two-stage exchange, Candida accounted for 7.2 % of infections, increasing to 9.4 % when adjusted with the current methodology (Lee et al., 2024). Multiple studies have demonstrated strong associations between Candida PJI and clinical factors characteristic of chronic refractory infection, including prolonged infection duration, multiple prior surgeries, obesity, diabetes mellitus, presence of a draining sinus, polymicrobial infection, and recent antibiotic exposure. The reported odds ratios range from 2.2 to 7.2 (Aunon et al., 2025; Ergin et al., 2024, 2025; Gross et al., 2021; Kuo et al., 2018; Luo et al., 2025; Riaz et al., 2020). These data collectively support an expected Candida proportion of approximately 10 % in chronic refractory PJI cases, the upper estimate of our quantitative analysis.
An important consideration is whether imputation of missing microbiologic data selectively inflates estimates of Candida involvement or instead reflects broader limitations in microbiologic characterization within published PJI series. Staphylococcal species, particularly Staphylococcus aureus and S. epidermidis, account for more than half of reported PJI cases and are more consistently identified and reported in routine clinical practice. However, even for bacterial pathogens, culture-negative and polymicrobial cases remain common, indicating that missingness reflects incomplete or heterogeneous microbiologic reporting rather than organism-specific bias. This analysis does not suggest that Candida replaces bacterial pathogens within culture-negative infections but rather that fungal involvement is under-recognized within a subset of these cases. Collectively, these findings underscore that missingness imputation does not “create” fungal infections but instead quantifies uncertainty arising from inconsistent application and reporting of microbiologic diagnostic methods.
This study has several limitations. The inability to stratify results by specific risk factors limits granularity and reflects a broader challenge in fungal PJI research: microbiologic data are inconsistently reported and often aggregated across heterogeneous patient populations. Additional limitations include reliance on retrospective studies, the relatively small cohort of confirmed fungal PJI cases, and the limited number of publications with sufficient detail to calculate risk ratios for each category of missing data. The imputed expected proportions represent extrapolations based on available information rather than true corrections derived from recovered data, and errors may be magnified in studies with multiple sources of missing data.
A quantitative sensitivity analysis of missing microbiologic data suggests that the contribution of Candida to PJI is likely underestimated. The expected proportion of Candida-associated PJI is at least 5 % of all confirmed PJI cases and closer to 10 % among patients with chronic refractory infections. The discrepancy between reported and expected incidence highlights the need for prospective, multi-center studies that incorporate high-sensitivity fungal diagnostics, including prolonged culture, molecular testing, and standardized reporting of polymicrobial infections. More accurate estimates of Candida involvement in PJI will strengthen risk stratification and better inform surgical and antimicrobial decision-making, particularly for patients with complex or treatment-resistant infection.
No data sets were used in this article.
SEO: conceptualization, data curation, investigation, formal analysis, methodology, validation, project administration, visualization, and writing (original draft and review and editing). AS: conceptualization, data curation, investigation, formal analysis, methodology, validation, project administration, visualization, and writing (original draft and review and editing). MFS: data curation, investigation, and project administration. VRW: data curation, investigation, and project administration. CFT: data curation, investigation, and project administration. CC: data curation, investigation, and project administration. NP: data curation, investigation, and project administration. BAK: supervision, project administration, resources, and writing (review and editing). JFP: supervision, project administration, resources, and writing (review and editing). NSP: supervision, project administration, resources, and writing (review and editing). KLU: conceptualization, methodology, supervision, investigation, project administration, resources, funding acquisition, and writing (original draft and review and editing).
Kenneth L. Urish serves as a consultant for Peptilogics, Smith & Nephew, Osteal Therapeutics, and Onkos Surgical. Johannes F. Plate and Brian A. Klatt are consultants for Smith & Nephew. Nicolas S. Piuzzi serves as a consultant for Zimmer and Stryker. One or more authors are members of the boards of the Musculoskeletal Infection Society (MSIS), ASTM International, American Academy of Orthopaedic Surgeons (AAOS), American Association of Hip and Knee Surgeons (AAHKS), the Eastern Orthopaedic Association (EOA), The Knee Society, Journal of Knee Surgery, and the Journal of Bone & Joint Surgery.
Ethical approval was not required, as this study analyzed previously published data only.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. The authors bear the ultimate responsibility for providing appropriate place names. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This research has been supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant no. R01AR082167).
This paper was edited by Derek Amanatullah and reviewed by two anonymous referees.
Alkhawashki, H., Benevenia, J., Drago, L., and Kadkoy, Y.: A One- or Two-Stage Revision of Fungal Prosthetic Joint Infection: A Review of Current Knowledge, Pitfalls and Recommendations, Antibiotics, 14, https://doi.org/10.3390/antibiotics14070658, 2025.
Aunon, A., Ortiz, I., Penarrubia, S., Alvaro, C., Torrecilla-Sadaba, E., Garcia-Canete, J., and Esteban, J.: Polymicrobial Prosthetic Joint Infections: Unraveling Risk Factors and Outcomes in a Single-Center Study, Microorganisms, 13, https://doi.org/10.3390/microorganisms13071679, 2025.
Baecker, H., Frieler, S., Gessmann, J., Pauly, S., Schildhauer, T. A., and Hanusrichter, Y.: Three-stage revision arthroplasty for the treatment of fungal periprosthetic joint infection: outcome analysis of a novel treatment algorithm: a prospective study, Bone Jt. Open, 2, 671–678, https://doi.org/10.1302/2633-1462.28.BJO-2021-0002.R2, 2021.
Benito, N., Franco, M., Ribera, A., Soriano, A., Rodriguez-Pardo, D., Sorli, L., Fresco, G., Fernandez-Sampedro, M., Dolores Del Toro, M., Guio, L., Sanchez-Rivas, E., Bahamonde, A., Riera, M., Esteban, J., Baraia-Etxaburu, J. M., Martinez-Alvarez, J., Jover-Saenz, A., Duenas, C., Ramos, A., Sobrino, B., Euba, G., Morata, L., Pigrau, C., Coll, P., Mur, I., Ariza, J., and REIPI (Spanish Network for Research in Infectious Disease) Group for the Study of Prosthetic Joint Infections: Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study, Clin. Microbiol. Infect., 22, 731–738, https://doi.org/10.1016/j.cmi.2016.05.004, 2016.
Brown, T. S., Petis, S. M., Osmon, D. R., Mabry, T. M., Berry, D. J., Hanssen, A. D., and Abdel, M. P.: Periprosthetic Joint Infection With Fungal Pathogens, J. Arthroplasty, 33, 2605–2612, https://doi.org/10.1016/j.arth.2018.03.003, 2018.
Budin, M., Sandiford, N. A., Gehrke, T., and Citak, M.: Body mass index matters: morbid obese patients have different microorganism profiles in the setting of periprosthetic hip joint infections, Int. Orthop., 49, 1309–1317, https://doi.org/10.1007/s00264-025-06513-4, 2025.
Cao, Q., Fan, P., Feng, J., Cheng, T., Wang, X., Cheng, C., and Dai, Z.: Comprehensive analysis of the pathogen spectrum and antibiotic resistance profiles in periprosthetic joint infections: a single center retrospective study, Front. Surg., 12, 1566689, https://doi.org/10.3389/fsurg.2025.1566689, 2025.
Del Pozo, J. L. and Patel, R.: Clinical practice. Infection associated with prosthetic joints, N. Engl. J. Med., 361, 787–794, https://doi.org/10.1056/NEJMcp0905029, 2009.
Enz, A., Mueller, S. C., Warnke, P., Ellenrieder, M., Mittelmeier, W., and Klinder, A.: Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint-A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence, J. Fungi, 7, https://doi.org/10.3390/jof7060404, 2021.
Ergin, M., Budin, M., Canbaz, S. B., Ciloglu, O., Gehrke, T., and Citak, M.: Microbiological profiles in periprosthetic joint infections after total knee arthroplasty: a comparative analysis of diabetic and non-diabetic patients, Int. Orthop., 48, 2633–2640, https://doi.org/10.1007/s00264-024-06275-5, 2024.
Ergin, M., Budin, M., Canbaz, S. B., Ciloglu, O., Salber, J., Gehrke, T., and Citak, M.: Microbial Diversity of Periprosthetic Joint Infections in Diabetic and Nondiabetic Patients Following Hip Arthroplasty, J. Arthroplasty, 40, 494–498, https://doi.org/10.1016/j.arth.2024.08.030, 2025.
Froschen, F. S., Randau, T. M., Franz, A., Molitor, E., and Hischebeth, G. T. R.: Microbiological Profiles of Patients with Periprosthetic Joint Infection of the Hip or Knee, Diagnostics, 12, https://doi.org/10.3390/diagnostics12071654, 2022.
Goswami, K., Clarkson, S., Phillips, C. D., Dennis, D. A., Klatt, B. A., O'Malley, M. J., Smith, E. L., Gililland, J. M., Pelt, C. E., Peters, C. L., Malkani, A. L., Palumbo, B. T., Lyons, S. T., Bernasek, T. L., Minter, J., Goyal, N., McDonald, J. F., 3rd, Cross, M. B., Prieto, H. A., Lee, G. C., Hansen, E. N., Bini, S. A., Ward, D. T., Shohat, N., Higuera, C. A., Nam, D., Della Valle, C. J., Parvizi, J., and Orthopedic Genomics Workgroup: An Enhanced Understanding of Culture-Negative Periprosthetic Joint Infection with Next-Generation Sequencing: A Multicenter Study, J. Bone. Joint Surg. Am., 104, 1523–1529, https://doi.org/10.2106/JBJS.21.01061, 2022.
Gross, C. E., Della Valle, C. J., Rex, J. C., Traven, S. A., and Durante, E. C.: Fungal Periprosthetic Joint Infection: A Review of Demographics and Management, J. Arthroplasty, 36, 1758–1764, https://doi.org/10.1016/j.arth.2020.11.005, 2021.
Haraldsdottir, I., Gunnlaugsdottir, S. L., Kristjansson, D. F., Erlendsdottir, H., Helgason, K. O., Gudbrandsson, E., Sigurdardottir, B., and Gottfredsson, M.: Changing Incidence, Aetiology and Outcomes of Prosthetic Joint Infections: A Population-Based Study in Iceland, J. Clin. Med., 14, https://doi.org/10.3390/jcm14155289, 2025.
Herndon, C. L., Rowe, T. M., Metcalf, R. W., Odum, S. M., Fehring, T. K., Springer, B. D., and Otero, J. E.: Treatment Outcomes of Fungal Periprosthetic Joint Infection, J. Arthroplasty, 38, e2431, https://doi.org/10.1016/j.arth.2023.05.009, 2023.
Higgins, J. P., White, I. R., and Wood, A. M.: Imputation methods for missing outcome data in meta-analysis of clinical trials, Clin. Trials, 5, 225–239, https://doi.org/10.1177/1740774508091600, 2008.
Jiang, G., Wang, W., Yang, Y., Zhang, M., Yang, Y., and Jiang, Q.: Organism profiles and empirical treatments for periprosthetic joint infections, J. Orthop. Surg. Res., 20, 698, https://doi.org/10.1186/s13018-025-06007-4, 2025.
Koutserimpas, C., Naoum, S., Giovanoulis, V., Raptis, K., Alpantaki, K., Dretakis, K., Vrioni, G., and Samonis, G.: Fungal Periprosthetic Hip Joint Infections, Diagnostics, 12, https://doi.org/10.3390/diagnostics12102341, 2022.
Kuo, F. C., Goswami, K., Shohat, N., Blevins, K., Rondon, A. J., and Parvizi, J.: Two-Stage Exchange Arthroplasty Is a Favorable Treatment Option Upon Diagnosis of a Fungal Periprosthetic Joint Infection, J. Arthroplasty, 33, 3555–3560, https://doi.org/10.1016/j.arth.2018.07.024, 2018.
Kurtz, S., Ong, K., Lau, E., Mowat, F., and Halpern, M.: Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030, J. Bone Joint Surg. Am., 89, 780–785, https://doi.org/10.2106/JBJS.F.00222, 2007.
Lee, Y., Lee, A., Jeong, H. S., Shin, S. U., Kim, U. J., Kim, S. E., Kang, S. J., Jung, S. I., Park, K. S., Seon, J. K., Shin, J. H., and Park, K. H.: The microbiology of periprosthetic joint infections as revealed by sonicate cultures in Korea: Routine use of fungal and mycobacterial cultures is necessary?, PLoS One, 19, e0309046, https://doi.org/10.1371/journal.pone.0309046, 2024.
Lin, L., Li, J., Zhang, C., Li, J., Wu, B., Huang, Z., Lv, J., Liu, M., Li, W., Zhang, W., and Fang, X.: Comprehensive analysis of culture-negative periprosthetic joint infection with metagenomic next-generation sequencing, Front. Cell Infect. Microbiol., 15, 1564488, https://doi.org/10.3389/fcimb.2025.1564488, 2025.
Luo, T. D., Budin, M., Karlidag, T., Lausmann, C., Gehrke, T., and Citak, M.: Risk Factors and Microbiological Profile of Knee Periprosthetic Joint Infections With Sinus Tract, J. Arthroplasty, 40, 214–217, https://doi.org/10.1016/j.arth.2024.06.062, 2025.
Lyu, J., Huang, J., Huang, J., Hu, H., Wang, Q., Ding, H., Li, H., Fang, X., and Zhang, W.: Rising challenges in periprosthetic joint infections: a focus on rare pathogens and their clinical implications, Front. Cell Infect. Microbiol., 14, 1451398, https://doi.org/10.3389/fcimb.2024.1451398, 2024.
McCulloch, R. A., Martin, A., Young, B. C., Kendrick, B. J., Alvand, A., Jeys, L., Stevenson, J., and Palmer, A. J.: Frequent microbiological profile changes are seen in subsequent-revision hip and knee arthroplasty for prosthetic joint infection, J. Bone Joint Infect., 8, 229–234, https://doi.org/10.5194/jbji-8-229-2023, 2023.
Mei, J., Hu, H., Zhu, S., Ding, H., Huang, Z., Li, W., Yang, B., Zhang, W., and Fang, X.: Diagnostic Role of mNGS in Polymicrobial Periprosthetic Joint Infection, J. Clin. Med., 12, https://doi.org/10.3390/jcm12051838, 2023.
Morreel, E. R. L., van Dessel, H. A., Geurts, J., Savelkoul, P. H. M., and van Loo, I. H. M.: Prolonged incubation time unwarranted for acute periprosthetic joint infections, J. Clin. Microbiol., 63, e0114324, https://doi.org/10.1128/jcm.01143-24, 2025.
Riaz, T., Tande, A. J., Steed, L. L., Demos, H. A., Salgado, C. D., Osmon, D. R., and Marculescu, C. E.: Risk Factors for Fungal Prosthetic Joint Infection, J. Bone Joint Infect., 5, 76–81, https://doi.org/10.7150/jbji.40402, 2020.
Sambri, A., Zunarelli, R., Fiore, M., Bortoli, M., Paolucci, A., Filippini, M., Zamparini, E., Tedeschi, S., Viale, P., and De Paolis, M.: Epidemiology of Fungal Periprosthetic Joint Infection: A Systematic Review of the Literature, Microorganisms, 11, https://doi.org/10.3390/microorganisms11010084, 2022.
Sidhu, M. S., Cooper, G., Jenkins, N., Jeys, L., Parry, M., and Stevenson, J. D.: Prosthetic fungal infections: poor prognosis with bacterial co-infection, Bone Joint J., 101-B, 582–588, https://doi.org/10.1302/0301-620X.101B5.BJJ-2018-1202.R1, 2019.
Tai, D. B. G., Patel, R., Abdel, M. P., Berbari, E. F., and Tande, A. J.: Microbiology of hip and knee periprosthetic joint infections: a database study, Clin. Microbiol. Infect., 28, 255–259, https://doi.org/10.1016/j.cmi.2021.06.006, 2022a.
Tai, D. B. G., Wengenack, N. L., Patel, R., Berbari, E. F., Abdel, M. P., and Tande, A. J.: Fungal and mycobacterial cultures should not be routinely obtained for diagnostic work-up of patients with suspected periprosthetic joint infections, Bone Joint J., 104-B, 53–58, https://doi.org/10.1302/0301-620X.104B1.BJJ-2021-0876.R1, 2022b.
Tande, A. J. and Patel, R.: Prosthetic joint infection, Clin. Microbiol. Rev., 27, 302–345, https://doi.org/10.1128/CMR.00111-13, 2014.
Tsai, Y., Chang, C. H., Lin, Y. C., Lee, S. H., Hsieh, P. H., and Chang, Y.: Different microbiological profiles between hip and knee prosthetic joint infections, J. Orthop. Surg., 27, 2309499019847768, https://doi.org/10.1177/2309499019847768, 2019.
Ull, C., Yilmaz, E., Baecker, H., Schildhauer, T. A., Waydhas, C., and Hamsen, U.: Microbial findings and the role of difficult-to-treat pathogens in patients with periprosthetic infection admitted to the intensive care unit, Orthop. Rev., 12, 8867, https://doi.org/10.4081/or.2020.8867, 2020.
van den Bijllaardt, W., van der Jagt, O. P., Peijs, M., Janssens, M., Buiting, A. G., and Reuwer, A. Q.: Culturing periprosthetic tissue in blood culture bottles results in isolation of additional microorganisms, Eur. J. Clin. Microbiol. Infect. Dis., 38, 245–252, https://doi.org/10.1007/s10096-018-3420-6, 2019.
Watanabe, S., Kamono, E., Choe, H., Ike, H., Inaba, Y., and Kobayashi, N.: Differences in Diagnostic Sensitivity of Cultures Between Sample Types in Periprosthetic Joint Infections: A Systematic Review and Meta-Analysis, J. Arthroplasty, 39, 1939–1945, https://doi.org/10.1016/j.arth.2024.03.016, 2024.
Wu, B., Lin, Y., Su, J., Lin, L., Yu, Z., Zhang, C., Fang, X., Huang, Z., and Zhang, W.: Extending the culture duration could not improve the culture positivity rate and clinical outcomes of periprosthetic joint infection, Front. Cell Infect. Microbiol., 15, 1551862, https://doi.org/10.3389/fcimb.2025.1551862, 2025.
Yang, C., Ji, B., Li, G., Zhang, X., Xu, B., and Cao, L.: Ninety-day postoperative mortality and complications in continuous and unselected single-stage revisions for chronic periprosthetic joint infection, Int. Orthop., 48, 1691–1700, https://doi.org/10.1007/s00264-024-06152-1, 2024.