the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Serum inflammatory markers for the screening and diagnosis of periprosthetic joint infection: a systematic review and meta-analysis
Irene K. Sigmund
Matthew J. Dietz
Marta Sabater-Martos
Antony J. R. Palmer
Nicolas Cortés-Penfield
Aim: As part of a multi-society effort to derive a unified consensus definition of periprosthetic joint infection (PJI), a systematic review of serum inflammatory marker diagnostic performance for hip, knee, and shoulder PJI was performed. Methods: PubMed (MEDLINE) and EMBASE were searched for studies reporting the diagnostic performance of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), fibrinogen, interleukin-6 (IL-6), or D-dimer for PJI. From these, each markers' pooled sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), and area under the summary receiver operating characteristic curve (AUSROC) were calculated using a random-effects model. Results: A total of 89 studies reported all diagnostic performance measures for at least one marker. CRP (84 studies, 22 351 patients) demonstrated a pooled sensitivity, specificity, PPV, NPV, and AUSROC of 79.4 % (95 %CI: 78.5–80.3), 77.7 % (77.1–78.3), 67.0 % (63.3–70.7), 86.6 % (84.5–88.7), and 0.872 (SE 0.01), respectively. Corresponding performance estimates for fibrinogen (14 studies, 3433 patients) were 70.9 % (68.3–73.3), 85.9 % (84.3–87.3), 77.2 % (71.8–82.6), 82.1 % (77.1–87.2), and 0.889 (0.02), respectively, and those for IL-6 (20 studies, 2318 patients) were 76.3 % (73.4–79.0), 85.8 % (83.8–87.6), 74.5 % (69.0–80.0), 86.0 % (80.6–91.3), and 0.900 (0.01), respectively. ESR, D-dimer, and WBC did not offer greater predictive values than these markers. Conclusion: Although serum CRP, fibrinogen, and IL-6 demonstrated the best performance among all analysed parameters, their diagnostic accuracy remains insufficient to reliably confirm or exclude PJI. Elevated serum markers should be re-evaluated as a diagnostic criterion in future PJI definitions. Level of evidence: The level of evidence was Level III.
- Article
(1225 KB) - Full-text XML
-
Supplement
(775 KB) - BibTeX
- EndNote
Serum inflammatory markers are widely used to aid the diagnosis of periprosthetic joint infection (PJI). They are easy to obtain, available globally, cheap, and deliver timely results. However, the accuracies of serum inflammatory markers for PJI are limited by their poor differentiation between septic and aseptic failure after total joint arthroplasty, particularly among patients with underlying inflammatory disorders.
Which (or whether) serum inflammatory markers should be used for PJI diagnosis remains unclear. The 2019 guideline of the American Association of Orthopaedic Surgeons (AAOS) recommends serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin 6 (IL-6) in the preoperative evaluation of PJI (Aaos, 2019). The 2018 revised International Consensus Meeting (ICM) definition (Shohat et al., 2019) includes elevated CRP, ESR, and D-dimer as minor diagnostic criteria. The Musculoskeletal Infection Society (MSIS) definitions from 2011 and 2013 (Parvizi et al., 2011; Parvizi and Gehrke, 2014) also incorporate CRP and ESR as minor criteria. In contrast, the 2021 definition proposed by the European Bone and Joint Infection Society (EBJIS) includes only serum CRP as a serum biomarker (McNally et al., 2021). Notably, the Infectious Disease Society of America (IDSA) guideline does not include any serum markers in its diagnostic criteria for PJI infection definition (Osmon et al., 2013).
The EBJIS, ICM, MSIS, IDSA, and the European Society of Clinical Microbiology and Infectious Diseases Study Group for Implant-Associated Infections (ESCMID-ESGIAI) have recently convened a joint taskforce to develop a unified consensus definition of PJI. As part of that effort, a systematic review and meta-analysis was conducted to assess the performance of sufficiently studied serum inflammatory markers for the preoperative diagnosis of PJI.
On behalf of the “Serum Marker Workgroup” of the Unified PJI Definition Taskforce, a systematic review and meta-analysis of the value of serum inflammatory markers for the diagnosis of hip, knee, and/or shoulder PJI was performed based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). The pooled accuracy estimates of each sufficiently studied serum inflammatory parameter were calculated, and markers were intercompared to find those most accurate. In addition, the best thresholds to differentiate between septic and aseptic arthroplasty failure for each serum parameter were evaluated. Given the great between-study heterogeneity in reference standards for infection, specific patient populations studied, and diagnostic thresholds for positivity in this literature base, only serum inflammatory markers evaluated in at least 10 studies on their diagnostic value in periprosthetic joint infection (PJI) were considered sufficiently studied and included in this systematic review and meta-analysis.
2.1 Search strategy and screening
Search queries for PubMed (MEDLINE) and EMBASE were developed to capture all clinical studies reporting diagnostic performance measures of one or more serum inflammatory markers for PJI (see Table S1 in the Supplement for full queries). The serum markers included in the search query were as follows: ESR; CRP; IL-6; D-dimer; fibrinogen; white blood cell count (WBC); procalcitonin; platelet volume; and the various ratios involving serum neutrophils, lymphocytes, monocytes, and/or immune globulins. The literature search was conducted on 1 February 2024. All retrieved titles and abstracts were divided into three equal sets, with each set screened once for eligibility by one of three authors (Irene K. Sigmund, Matthew J. Dietz, and Nicolas Cortés-Penfield). The resulting eligible studies were then again divided into three sets, and each author reviewed the full texts of their assigned set once, applying the predefined inclusion and exclusion criteria and documenting reasons for exclusion. Additionally, relevant systematic reviews identified in the search were screened to identify any further eligible studies not captured initially.
2.2 Inclusion and exclusion criteria
English-language studies assessing the diagnostic value of at least one serum inflammatory marker in diagnosing PJI after a total hip, knee, or shoulder arthroplasty were considered for inclusion. Further inclusion criteria were that (1) the study clearly stated the reference standard for PJI diagnosis and (2) it reported the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for at least one serum inflammatory marker. Studies including infections other than PJI, case reports, and non-human studies were excluded.
2.3 Data extraction
Relevant study characteristics and diagnostic accuracy data (i.e. sensitivity, specificity, PPV, and NPV) from each eligible study were extracted by three authors (Irene K. Sigmund, Matthew J. Dietz, and Nicolas Cortés-Penfield) using a standardized form. Relevant study characteristics included the PJI reference standard; number of patients with an affected knee, hip, or shoulder; number of patients with PJI and aseptic failure; and the cutoff value for an abnormal test result used as well as the origin of that cutoff (i.e. prespecified vs. derived post hoc from the Youden index).
2.4 Quality assessment
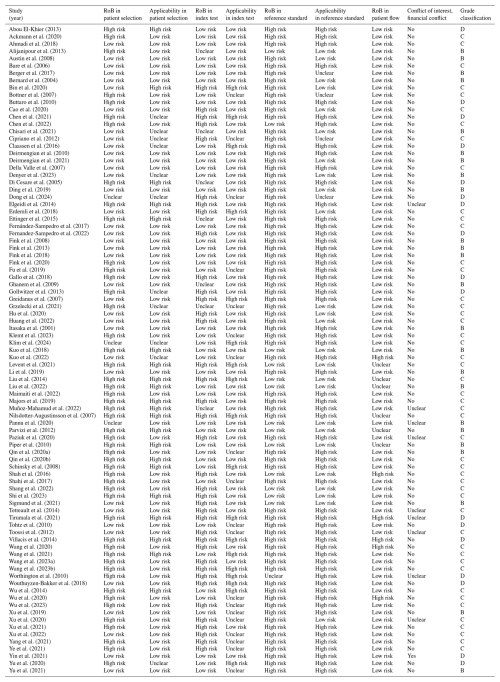
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was used to assess the risk of bias and applicability to this meta-analysis across four domains, including patient selection, index test, reference standard, and enrolment flow of patients/timing of the index and reference tests (Whiting et al., 2011). Risk of bias and applicability were rated as “low”, “high”, or “unclear” based on the provided study information, and the quality of each included study was then graded from A (high) to D (very low). Quality assessment was performed by the author assigned to each specific article during the full-text review and data extraction. Each study was reviewed by a single author. In cases of uncertainty or ambiguity, a second author re-reviewed the article, and any discrepancies were resolved through discussion to reach a consensus.
2.5 Statistical analysis
MetaDiSc 1.4® and RStudio 4.4.1® were used for quantitative analyses of each serum inflammatory biomarker. The number of true positives, false positives, true negatives, and false negatives for each serum inflammatory parameter were calculated using the number of PJI and aseptic cases and the given sensitivities and specificities in each study. The pooled estimates for sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR−), and the area under the summary receiver operating characteristic curve (AUSROC) were calculated with their 95 % confidence intervals (CIs). The diagnostic odds ratio (DOR) was evaluated to measure the effectiveness of diagnostic testing. Statistical heterogeneity for each accuracy measure was determined by Higgins I2 statistic (I2 values > 50 % reflect substantial heterogeneity and should be interpreted with caution; Higgins and Thompson, 2002). Scatterplots comparing Youden indices with cutoffs were used to determine the optimal cutoff for each parameter using jamovi (version 2.2.5). For parameters providing the required data, the optimal cutoff for only hips or only knees was calculated. Due to the limited number of data for shoulders, no detailed analyses could be performed.
3.1 Study identification and inclusion
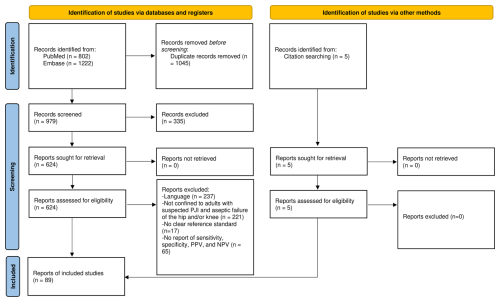
The initial search yielded 802 results from PubMed and 1222 results from EMBASE. After automated reference extraction with the removal of missing and duplicate references using EndNote and the elimination of irrelevant references via title screening, 624 references underwent abstract screening. After applying the inclusion and exclusion criteria and screening the references of prior relevant systematic reviews identified in the search, we included a total 89 studies in this meta-analysis (Fig. 1).
Of the serum inflammatory markers included in the search query, only CRP, ESR, WBC, fibrinogen, IL-6, and D-dimer met the criteria for inclusion in our meta-analysis. Serum procalcitonin, platelet volume, and ratios of specific cell lines and/or immune globulins all had ≤ 10 included studies reporting their accuracy in PJI diagnosis and were not considered further.
In total, 84 studies (including 22 351 patients) evaluated the diagnostic potential of serum CRP (Abou El-Khier et al., 2013; Ackmann et al., 2020; Ahmadi et al., 2018; Alijanipour et al., 2013; Austin et al., 2008; Bare et al., 2006; Berger et al., 2017; Bernard et al., 2004; Bin et al., 2020; Bottner et al., 2007; Buttaro et al., 2010; Cao et al., 2020; Chen et al., 2021; Chisari et al., 2021; Cipriano et al., 2012; Claassen et al., 2016; Deirmengian et al., 2010; Deirmengian et al., 2021; Della Valle et al., 2007; Denyer et al., 2023; Di Cesare et al., 2005; Ding et al., 2019; Dong et al., 2024; Elgeidi et al., 2014; Erdemli et al., 2018; Ettinger et al., 2015; Fernández-Sampedro et al., 2017; Fernandez-Sampedro et al., 2022; Fink et al., 2008; Fink et al., 2013; Fink et al., 2018; Fink et al., 2020; Fu et al., 2019; Ghanem et al., 2009; Greidanus et al., 2007; Grzelecki et al., 2021; Hu et al., 2020; Huang et al., 2022; Itasaka et al., 2001; Klemt et al., 2023; Klim et al., 2024; Kuo et al., 2018; Kuo et al., 2022; Levent et al., 2021; Li et al., 2019; Liu et al., 2014; Liu et al., 2022; Maimaiti et al., 2022; Majors and Jagadale, 2019; Muñoz-Mahamud et al., 2022; Nilsdotter-Augustinsson et al., 2007; Parvizi et al., 2012; Paziuk et al., 2020; Piper et al., 2010; Qin et al., 2020a; Qin et al., 2020b; Schinsky et al., 2008; Shah et al., 2016; Shahi et al., 2017; Shang et al., 2022; Shi et al., 2023; Sigmund et al., 2021; Tetreault et al., 2014; Tirumala et al., 2021; Tohtz et al., 2010; Villacis et al., 2014; Wang et al., 2020, 2021, 2023a, b; Worthington et al., 2010; Wouthuyzen-Bakker et al., 2018; Wu et al., 2014, 2020, 2023; Xu et al., 2019, 2020, 2021, 2022; Yang et al., 2021; Ye et al., 2021; Yin et al., 2021; Yu et al., 2020, 2021), 67 studies (including 19660 patients) evaluated the diagnostic potential of ESR (Abou El-Khier et al., 2013; Ahmadi et al., 2018; Alijanipour et al., 2013; Austin et al., 2008; Bare et al., 2006; Berger et al., 2017; Bernard et al., 2004; Bottner et al., 2007; Buttaro et al., 2010; Chen et al., 2021; Chisari et al., 2021; Cipriano et al., 2012; Deirmengian et al., 2010; Deirmengian et al., 2021; Della Valle et al., 2007; Denyer et al., 2023; Di Cesare et al., 2005; Ding et al., 2019; Dong et al., 2024; Elgeidi et al., 2014; Fernandez-Sampedro et al., 2022; Fu et al., 2019; Ghanem et al., 2009; Greidanus et al., 2007; Grzelecki et al., 2021; Hu et al., 2020; Huang et al., 2022; Itasaka et al., 2001; Klemt et al., 2023; Kuo et al., 2018; Kuo et al., 2022; Li et al., 2019; Liu et al., 2014, 2022; Maimaiti et al., 2022; Majors and Jagadale, 2019; Muñoz-Mahamud et al., 2022; Nilsdotter-Augustinsson et al., 2007; Paziuk et al., 2020; Piper et al., 2010; Qin et al., 2020a, b; Schinsky et al., 2008; Shah et al., 2016; Shahi et al., 2017; Shang et al., 2022; Shi et al., 2023; Tirumala et al., 2021; Tohtz et al., 2010; Villacis et al., 2014; Wang et al., 2020, 2021, 2023a, 2023b; Worthington et al., 2010; Wouthuyzen-Bakker et al., 2018; Wu et al., 2014, 2020, 2023; Xu et al., 2019, 2020, 2021, 2022; Yang et al., 2021; Ye et al., 2021; Yu et al., 2020; Chen et al., 2022), 14 studies (including 3550 patients) evaluated the diagnostic potential of WBC (Abou El-Khier et al., 2013; Bottner et al., 2007; Di Cesare et al., 2005; Elgeidi et al., 2014; Itasaka et al., 2001; Klim et al., 2024; Li et al., 2019; Maimaiti et al., 2022; Sigmund et al., 2021; Tohtz et al., 2010; Toossi et al., 2012; Villacis et al., 2014; Yang et al., 2021; Yu et al., 2020), 14 studies (including 3433 patients) evaluated the diagnostic potential of fibrinogen (Bin et al., 2020; Chen et al., 2021, 2022; Chisari et al., 2021; Dong et al., 2024; Li et al., 2019; Maimaiti et al., 2022; Sigmund et al., 2021; Wang et al., 2020; Wu et al., 2020, 2023; Xu et al., 2020, 2022; Yang et al., 2021), 20 studies (including 2318 patients) evaluated the diagnostic potential of IL-6 (Abou El-Khier et al., 2013; Ackmann et al., 2020; Bottner et al., 2007; Buttaro et al., 2010; Di Cesare et al., 2005; Elgeidi et al., 2014; Erdemli et al., 2018; Ettinger et al., 2015; Gallo et al., 2018; Gollwitzer et al., 2013; Majors and Jagadale, 2019; Qin et al., 2020b; Villacis et al., 2014; Worthington et al., 2010; Xu et al., 2019, 2021, 2022; Yin et al., 2021; Yu et al., 2020, 2021), and 21 studies (including 3177 patients) evaluated the diagnostic potential of D-dimer (Ackmann et al., 2020; Chen et al., 2021; Chisari et al., 2021; Dong et al., 2024; Fernandez-Sampedro et al., 2022; Fu et al., 2019; Grzelecki et al., 2021; Hu et al., 2020; Kuo et al., 2022; Li et al., 2019; Liu et al., 2022; Maimaiti et al., 2022; Muñoz-Mahamud et al., 2022; Shahi et al., 2017; Wu et al., 2020; Xu et al., 2019, 2021; Chen et al., 2022; Pannu et al., 2020; Qin et al., 2020a; Wang et al., 2020). Full characteristics of all included studies are provided in Tables S2–S7.
3.2 Quality assessment
Of the 89 included studies, 24 were graded as moderate (B), 48 as low (C), and 17 as very low (D) using the QUADAS-2 risk of bias assessment. No study was graded as high quality (A). Risk of bias and applicability concerns are shown in Table 1. In the majority of studies (84 % n= 75/89), the overall risk of bias regarding the reference standard was high, with significant concerns about the applicability of the reference standard in 67 % of studies (n= 60/89). Incorporation bias (i.e. use of PJI diagnostic criteria that include the inflammatory markers being studied as the reference standard) was a consistent issue with these studies and may have inflated the apparent accuracy of ESR, CRP, and/or D-dimer relative to other markers.
3.3 Diagnostic potential of serum inflammatory markers
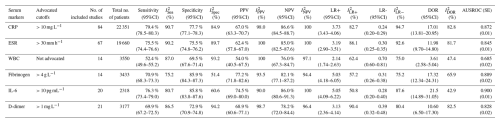
The detailed performance estimates of serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), fibrinogen, interleukin-6, and D-dimer are illustrated in Table 2. Summarized receiver operating curves of all serum parameters are illustrated in Fig. 2. The highest Youden index was observed at cutoffs of 10.7 mg L−1 (95 %CI: 9.8–11.6) for CRP, 30.6 mm h−1 (29.2–32.0) for ESR, 9.13 G L−1 (8.3–10.0) for WBC, 3.8 g L−1 (3.7–4.0) for fibrinogen, 9.8 pg mL−1 (7.8–11.8) for IL-6, and 1.1 mg L−1 (0.8–1.4) for D-dimer. However, all parameters demonstrated a low Spearman correlation (< 0.6) without a significant threshold effect. Meta-regression revealed that the diagnostic threshold used for ESR and CRP is not associated with diagnostic performance.
Table 2Pooled sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR−), diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic curve (AUSROC) for serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), fibrinogen, interleukin-6, and D-dimer.
Other terms used in the table are as follows: CI – confidence interval; I2 – I square, inconsistency.
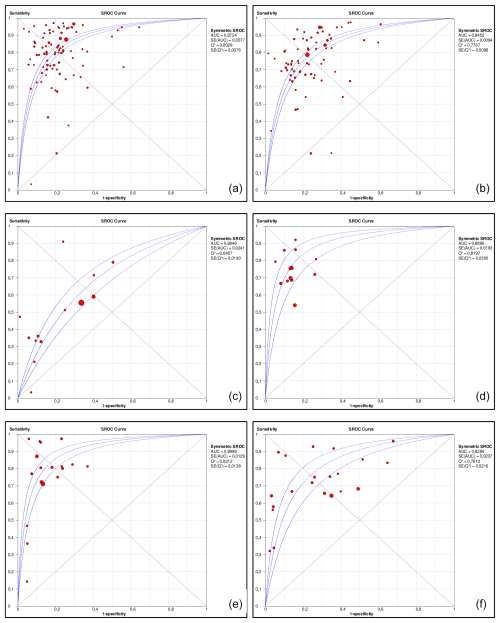
Figure 2Area under the summary receiver operating characteristic curves (AUSROCs) for C-reactive protein (CRP, a), erythrocyte sedimentation rate (ESR, b), white blood cell count (WBC, c), fibrinogen (d), interleukin-6 (e), and D-dimer (f).
Among the analysed serum inflammatory markers, CRP, fibrinogen, and IL-6 demonstrated the best performance for diagnosing PJI. Serum CRP offered superior sensitivity compared to fibrinogen (79 % [95 %CI 78.5–80.3] vs. 71 % [95 %CI 68.3–73.3]) but inferior specificity (77.7 % [77.1–78.3] vs. 85.9 % [84.3–87.3]). IL-6 also offered superior sensitivity compared to fibrinogen (76.3 % [73.4–79.0] vs. 69.9 % [67.2–72.5]) but with comparable specificity (85.8 % [83.8–87.6] vs. 85.9 % [84.3–87.3]). ESR sensitivity was inferior to CRP, comparable to IL-6, and slightly superior to fibrinogen, but its specificity was inferior to all three, making it no better as a rule-out test and less useful as a rule-in test vs. the aforementioned comparators. As for the other inflammatory markers, D-dimer demonstrated sensitivity similar to fibrinogen and inferior to alternatives, with specificity similar to ESR and inferior to alternatives, while serum WBC demonstrated little to no diagnostic utility. I2 values for nearly every diagnostic parameter for every marker exceeded 50 %, suggesting substantial between-study heterogeneity.
Only CRP and ESR had sufficient studies reporting their diagnostic utility in hip vs. knee PJI to facilitate stratified analyses. CRP appeared modestly less sensitive for knee vs. hip PJI (75.9 % [95 %CI 73.0–78.7] vs. 83.1 % [95 %CI: 79.8–86.0]) but similarly specific, with a comparable overall AUSROC (0.848 [SE 0.01] vs. 0.850 [SE 0.03]) and optimal diagnostic thresholds (9.1 mg L−1 [7.1–11.2] vs. 11.1 mg L−1 [8.9–13.3]). ESR appeared similar in both sensitivity (79.9 % [75.9–83.4] vs. 84.4 % [82.1–87.2]) and specificity (69.9 % [67.1–72.7] vs. 71.6 % [68.9–74.2]) for hip vs. knee PJI, with comparable optimal diagnostic thresholds (30 mm h−1 [30–30] vs. 28.8 mm h−1 [26.9–30.8]). These findings need to be interpreted with caution due to the small number of studies included and the heterogeneity of inclusion and exclusion criteria of the analysed studies. No marker had sufficient studies reporting accuracy in shoulder PJI for a stratified analysis.
Reporting of PJI timing and/or chronicity (i.e. early postoperative vs. acute hematogenous vs. chronic PJI) was limited and inconsistent across the identified studies; we judged the data insufficient to derive optimal diagnostic thresholds of any inflammatory marker for acute PJI. Similarly, data were insufficient to perform sub-analyses of markers' relative diagnostic utilities in specific patient populations (e.g. those with underlying inflammatory disorders).
Among the analysed serum inflammatory markers, CRP, fibrinogen, and IL-6 demonstrated the best performance for diagnosing PJI and were largely equivalent with respect to performance. Serum CRP offered a slightly superior sensitivity (79 %) compared to fibrinogen (71 %) and IL-6 (76 %) but inferior specificity (CRP: 78 %; fibrinogen: 86 %; IL-6: 86 %), leading to a lower PPV and similar NPV. Relative to CRP and fibrinogen, IL-6 is less widely available as an in-house test and may be more expensive in comparison to the other serum parameters.
While ESR has a long history of use in PJI diagnosis and was incorporated into previous PJI diagnostic criteria, its relative lack of specificity translated into an NPV that was no better than that for CRP, fibrinogen, or IL-6 and a similar or worse PPV. Serum WBC was not valuable as a diagnostic tool, nor was D-dimer, which is incorporated into the 2018 ICM criteria for PJI and was inferior with respect to sensitivity and/or specificity to all other serum biomarkers studied. We, therefore, recommend future definitions do not use WBC, ESR, or D-dimer as criteria.
Although a cutoff of 10.7 mg L−1 for CRP demonstrated the best Youden index in our scatterplot analysis, it was not substantially superior to the established cutoff of 10 mg L−1 (despite post hoc derivations of optimal cutoffs likely overestimating their true utility). In meta-regression analysis, the diagnostic threshold used for ESR and CRP in each study was not associated with diagnostic performance. For fibrinogen and IL-6, no consensus cutoff value was consistently used among the included studies, and optimal Youden indices were observed with cutoffs of 3.8 g L−1 and 9.8 pg mL−1, respectively. Given that most studies used a diagnostic threshold of 10 mg L−1 for CRP and that alternative threshold did not improve accuracy, we recommend the adoption of this threshold. Based on our analysis, thresholds of > 10 mg L−1 for CRP, > 4 g L−1 for fibrinogen, and > 10 pg mL−1 for IL-6 can be proposed.
Data on the performance of the serum inflammatory parameters at one site (hip, knee, or shoulder) were only available for CRP and ESR. Compared to knees, hips offered a substantially superior sensitivity (83.1 % vs. 75.9 %) and similar specificity (76.6 % vs. 76.8 %) of CRP. Hence, it seems that CRP performs better in hips. Interestingly, the opposite was observed when analysing ESR; knees demonstrated a slightly superior sensitivity (84.4 % vs. 79.9 %) and equivalent specificity (71.6 % vs. 69.9 %). However, these findings need to be interpreted with caution due to the small number of studies included and the heterogeneity in the inclusion and exclusion criteria of the analysed studies. Based on these findings, no definitive conclusion regarding the affected joint can be drawn.
However, due to their overall insufficient diagnostic accuracy, all of these serum inflammatory markers cannot be considered reliable stand-alone tests for PJI. Given these limitations and the increasing evidence supporting the higher sensitivity and specificity of synovial vs. serum inflammatory markers for PJI, it is time to reconsider the inclusion of serum markers in future definitions of periprosthetic joint infection.
Due to the low sensitivities, a negative result of these markers cannot rule out PJI. The high number of false negatives can be explained by the inadequate immune response in PJIs caused by low-virulence microorganisms (i.e.; coagulase-negative staphylococci and Cutibacterium spp., Akgün et al., 2018) and in PJIs with draining sinus tracts. In addition, patients with an impaired immune system or under immunomodulatory or antimicrobial therapy may also have normal concentrations, even if an infection is present. On the other hand, the elevation of these serum markers cannot confirm an infection and, therefore, cannot be recommended as stand-alone test for diagnosing PJI. The high false-positive rate can be explained by the fact that they are systemic parameters influenced by other inflammatory conditions, such as autoimmune disorders (i.e. rheumatoid arthritis, psoriasis, or systemic lupus erythematosus), active cancer, or other infectious foci at another site (i.e. pneumonia or endocarditis). Due to these limitations, we propose that the role of serum inflammatory markers is to supplement other diagnostic tests, rather than to be used in isolation. More invasive investigations are needed to accurately diagnose PJI. We recommend obtaining synovial fluid analysis in all cases with suspected PJI, even if CRP, IL-6, and fibrinogen are normal.
The majority of included studies (n= 65/89, 73 %) were of low or very low quality. Only 24 studies (27 %) were of moderate quality and none were of high quality. Significant limitations of this meta-analysis include the different infection definitions utilized, the heterogeneity of inclusion and exclusion criteria, the wide variability in applied cutoffs, incorporation bias (for CRP, ESR, and D-dimer), high between-study heterogeneity across nearly all performance measures of all markers, and limited information on test reproducibility. Almost all studies failed to differentiate between the performance of serum inflammatory parameters in acute (early postoperative/early acute and acute haematogenous/late acute) vs. chronic infections and were limited to hip and knee PJI. Therefore, based on these data, it is unclear whether our recommendations can be generalized to periprosthetic shoulder infections (or other less common types of arthroplasty), in which the epidemiology and causative microorganisms differ. Lastly, some studies included not only patients with osteoarthritis but also those with rheumatoid arthritis and/or immunosuppression. However, due to the limited number of such cases, stratified analysis was not feasible.
Although serum CRP, fibrinogen, and interleukin-6 demonstrated the highest diagnostic performance among the analysed markers in our meta-analysis, their accuracy remains insufficient to recommend their use as stand-alone tests for diagnosing PJI. These markers may be useful as part of a broader diagnostic workup but should be interpreted in conjunction with other clinical and laboratory findings.
In light of these findings, future PJI definitions may consider placing less emphasis on serum biomarkers as primary diagnostic criteria, favouring more accurate modalities such as synovial fluid analysis. Nevertheless, serum biomarkers might retain value as adjunctive screening tools, particularly in early diagnostic stages. Importantly, these results support pursuing synovial fluid analysis regardless of normal serum marker levels.
All data generated or analysed in this position paper are included in the published article or in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/jbji-10-363-2025-supplement.
Marjan Wouthuyzen-Bakker (Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands), Thomas Bauer (Department of Pathology, the Cleveland Clinic Foundation, Cleveland, OH, USA), Elie Berbari (Division of Public Health, Infectious Diseases and Occupational Medicine, Department of Medicine, Mayo Clinic College of Medicine and Science, Mayo Clinic, Rochester, MN, USA), Martin Clauss (Department of Orthopaedics and Trauma Surgery, University Hospital Basel, Basel, Switzerland), Nicolas Cortes-Penfield (Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, NE, USA), Matthew Dietz (Department of Orthopaedics, School of Medicine, West Virginia University, Morgantown, WV, USA), Jaime Esteban (Department of Medical Microbiology, IIS-Fundacion Jimenez Diaz, Madrid, Spain), Tristan Ferry (Infectious Diseases Department, Croix-Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Thorsten Gehrke (Department of Orthopaedic Surgery, ENDO-Klinik Hamburg, Specialist Clinic for Bone and Joint Surgery, Hamburg, Germany), Andor Glaudemans (Medical Imaging Center, Department of Nuclear Medicine & Molecular Imaging, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands), Benjamin Langworth (Division of Biostatistics and Health Data Science, School of Public Health, University of Minnesota, Rochester, MN, USA), Martin McNally (the Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford University Hospitals, Oxford, UK), Andy Miller (Department of Infectious Disease, Hospital for Special Surgery, New York, NY, USA), Sandra Nelson (Division of Infectious Diseases, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA), Javad Parvizi (Department of Orthopaedic Surgery, International Joint Center, Acibadem University Hospital, Istanbul, Türkiye), Robin Patel (Division of Infectious Diseases, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA), Holger Rohde (Institute of Medical Microbiology, Virology and Hygiene, University Medical Center Hamburg-Eppendorf, Hamburg, Germany), Thorsten Seyler (Department of Orthopaedics, Duke University Hospital, Durham, NC, USA), Irene Sigmund (Department of Orthopaedics and Trauma Surgery, Medical University of Vienna, Vienna, Austria), Alex Soriano (Department of Infectious Diseases, University of Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer, Hospital Clinic of Barcelona, CIBERINF Ciber in Infectious Diseases, Barcelona, Spain), and Ricardo Sousa (Department of Orthopedics, Centro Hospitalar Universitário de Santo António, Porto, Portugal) on behalf of the “Serum Marker Workgroup” for the Unified PJI Definition Taskforce.
IKS: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft, and writing – review and editing; MJD: conceptualization, data curation, investigation, methodology, supervision, and writing – review and editing; MSM: formal analysis, writing – original draft, and writing – review and editing; AJRP: formal analysis and writing – review and editing; NCP: conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, visualization, writing – original draft, and writing – review and editing.
At least one of the (co-)authors is a member of the editorial board of Journal of Bone and Joint Infection. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
No ethical approval was needed, as data from previous published studies were retrieved and analysed.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This paper was edited by Gina Suh and reviewed by three anonymous referees.
Aaos: Diagnosis and prevention of periprosthetic joint infections: Clinical practice guideline: https://aaos.org/quality/quality-programs/tumor-infection-and-military-medicine-programs/diagnosis--prevention-of-periprosthetic-joint-infections/, 2019.
Abou El-Khier, N. T., El Ganainy Ael, R., Elgeidy, A., and Rakha, S. A.: Assessment of interleukin-6 and other inflammatory markers in the diagnosis of Egyptian patients with periprosthetic joint infection, Egypt J. Immunol., 20, 93–99, 2013.
Ackmann, T., Möllenbeck, B., Gosheger, G., Schwarze, J., Schmidt-Braekling, T., Schneider, K. N., Frommer, A., Dieckmann, R., and Theil, C.: Comparing the Diagnostic Value of Serum D-Dimer to CRP and IL-6 in the Diagnosis of Chronic Prosthetic Joint Infection, Journal of Clinical Medicine, 9, https://doi.org/10.3390/jcm9092917, 2020.
Ahmadi, S., Lawrence, T. M., Sahota, S., Schleck, C. D., Harmsen, W. S., Cofield, R. H., and Sperling, J. W.: Significance of Perioperative Tests to Diagnose the Infection in Revision Total Shoulder Arthroplasty, Arch. Bone Jt. Surg., 6, 359–364, 2018.
Akgün, D., Müller, M., Perka, C., and Winkler, T.: The serum level of C-reactive protein alone cannot be used for the diagnosis of prosthetic joint infections, especially in those caused by organisms of low virulence, The Bone & Joint Journal, 100-b, 1482–1486, https://doi.org/10.1302/0301-620x.100b11.Bjj-2018-0514.R1, 2018.
Alijanipour, P., Bakhshi, H., and Parvizi, J.: Diagnosis of periprosthetic joint infection: the threshold for serological markers, Clinical Orthopaedics and Related Research, 471, 3186–3195, https://doi.org/10.1007/s11999-013-3070-z, 2013.
Austin, M. S., Ghanem, E., Joshi, A., Lindsay, A., and Parvizi, J.: A simple, cost-effective screening protocol to rule out periprosthetic infection, The Journal of Arthroplasty, 23, 65–68, https://doi.org/10.1016/j.arth.2007.09.005, 2008.
Bare, J., MacDonald, S. J., and Bourne, R. B.: Preoperative evaluations in revision total knee arthroplasty, Clinical Orthopaedics and Related Research, 446, 40–44, https://doi.org/10.1097/01.blo.0000218727.14097.d5, 2006.
Berger, P., Van Cauter, M., Driesen, R., Neyt, J., Cornu, O., and Bellemans, J.: Diagnosis of prosthetic joint infection with alpha-defensin using a lateral flow device: a multicentre study, The Bone & Joint Journal, 99-b, 1176–1182, https://doi.org/10.1302/0301-620x.99b9.Bjj-2016-1345.R2, 2017.
Bernard, L., Lübbeke, A., Stern, R., Bru, J. P., Feron, J. M., Peyramond, D., Denormandie, P., Arvieux, C., Chirouze, C., Perronne, C., and Hoffmeyer, P.: Value of preoperative investigations in diagnosing prosthetic joint infection: retrospective cohort study and literature review, Scand. J. Infect. Dis., 36, 410–416, https://doi.org/10.1080/00365540410015240, 2004.
Bin, G., Xinxin, Y., Fan, L., Shenghong, W., and Yayi, X.: Serum Fibrinogen Test Performs Well for the Diagnosis of Periprosthetic Joint Infection, The Journal of Arthroplasty, 35, 2607–2612, https://doi.org/10.1016/j.arth.2020.04.081, 2020.
Bottner, F., Wegner, A., Winkelmann, W., Becker, K., Erren, M., and Götze, C.: Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement, The Journal of Bone and Joint Surgery, 89, 94-99, https://doi.org/10.1302/0301-620x.89b1.17485, 2007.
Buttaro, M. A., Tanoira, I., Comba, F., and Piccaluga, F.: Combining C-reactive protein and interleukin-6 may be useful to detect periprosthetic hip infection, Clinical Orthopaedics and Related Research, 468, 3263–3267, https://doi.org/10.1007/s11999-010-1451-0, 2010.
Cao, H., Deng, P., Ye, P., Jie, K., Zeng, J., Feng, W., Chen, J., Qi, X., Li, J., Tan, X., Zhang, H., and Zeng, Y.: Platelet count as a novel potential predictor of periprosthetic joint infection, Chin. J. Tissue Eng. Res., 24(35), 5635–5640, 2020.
Chen, X., Qian, W., Weng, X., Lin, J., Jin, J., Wang, Y., and Zhu, S.: Different diagnostic performance of plasma fibrinogen and D-dimer in periprosthetic joint infection: a propensity score matched study, BMC Musculoskeletal Disorders, 22, 422, https://doi.org/10.1186/s12891-021-04282-w, 2021.
Chen, Y., Wang, H., Chen, X., Ma, H., Zheng, J., and Cao, L.: Serum D-lactate, a novel serological biomarker, is promising for the diagnosis of periprosthetic joint infection, BMC Musculoskeletal Fisorders, 23, 292, https://doi.org/10.1186/s12891-022-05199-8, 2022.
Chisari, E., Yacovelli, S., Goswami, K., Shohat, N., Woloszyn, P., and Parvizi, J.: Leukocyte Esterase Versus ICM 2018 Criteria in the Diagnosis of Periprosthetic Joint Infection, The Journal of Arthroplasty, 36, 2942–2945, https://doi.org/10.1016/j.arth.2021.03.006, 2021.
Cipriano, C. A., Brown, N. M., Michael, A. M., Moric, M., Sporer, S. M., and Della Valle, C. J.: Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis, The Journal of Bone and Joint Surgery, 94, 594–600, https://doi.org/10.2106/jbjs.j.01318, 2012.
Claassen, L., Ettinger, S., Pastor, M. F., Budde, S., Windhagen, H., and Floerkemeier, T.: The value of arthroscopic neosynovium biopsies to diagnose periprosthetic knee joint low-grade infection, Archives of orthopaedic and traumatic surgery, Archiv fur orthopadische und Unfall-Chirurgie, 136, 1753–1759, https://doi.org/10.1007/s00402-016-2574-x, 2016.
Deirmengian, C., Hallab, N., Tarabishy, A., Della Valle, C., Jacobs, J. J., Lonner, J., and Booth Jr., R. E.: Synovial fluid biomarkers for periprosthetic infection, Clinical Orthopaedics and Related Research, 468, 2017–2023, https://doi.org/10.1007/s11999-010-1298-4, 2010.
Deirmengian, C., Madigan, J., Kallur Mallikarjuna, S., Conway, J., Higuera, C., and Patel, R.: Validation of the Alpha Defensin Lateral Flow Test for Periprosthetic Joint Infection, The Journal of Bone and Joint Surgery, 103, 115–122, https://doi.org/10.2106/jbjs.20.00749, 2021.
Della Valle, C. J., Sporer, S. M., Jacobs, J. J., Berger, R. A., Rosenberg, A. G., and Paprosky, W. G.: Preoperative testing for sepsis before revision total knee arthroplasty, The Journal of Arthroplasty, 22, 90–93, https://doi.org/10.1016/j.arth.2007.04.013, 2007.
Denyer, S., Eikani, C., Sheth, M., Schmitt, D., and Brown, N.: Diagnosing periprosthetic joint infection, Bone Jt. Open, 4, 881–888, https://doi.org/10.1302/2633-1462.411.Bjo-2023-0094.R1, 2023.
Di Cesare, P. E., Chang, E., Preston, C. F., and Liu, C. J.: Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty, The Journal of Bone and Joint Surgery, 87, 1921–1927, https://doi.org/10.2106/jbjs.D.01803, 2005.
Ding, B. T., Tan, K. G., Kau, C. Y., Chan, H. Y. H., and Mohd Fadil, M. F. B.: Accuracy of the α-defensin lateral flow assay for diagnosing periprosthetic joint infection in Asians, Journal of Orthopaedic Surgery, 27, 2309499019828459, https://doi.org/10.1177/2309499019828459, 2019.
Dong, M., Wang, Y., Fan, H., Yang, D., Wang, R., and Feng, Y.: The Albumin to Globulin Ratio Performs Well for Diagnosing Periprosthetic Joint Infection: A Single-Center Retrospective Study, The Journal of Arthroplasty, 39, 229–235, https://doi.org/10.1016/j.arth.2023.08.002, 2024.
Elgeidi, A., Elganainy, A. E., Abou Elkhier, N., and Rakha, S.: Interleukin-6 and other inflammatory markers in diagnosis of periprosthetic joint infection, International Orthopaedics, 38, 2591–2595, https://doi.org/10.1007/s00264-014-2475-y, 2014.
Erdemli, B., Özbek, E. A., Başarir, K., Karahan, Z. C., Öcal, D., and Biriken, D.: Proinflammatory biomarkers' level and functional genetic polymorphisms in periprosthetic joint infection, Acta orthopaedica et traumatologica turcica, 52, 143–147, https://doi.org/10.1016/j.aott.2017.11.002, 2018.
Ettinger, M., Calliess, T., Kielstein, J. T., Sibai, J., Brückner, T., Lichtinghagen, R., Windhagen, H., and Lukasz, A.: Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure, Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 61, 332–341, https://doi.org/10.1093/cid/civ286, 2015.
Fernández-Sampedro, M., Fariñas-Alvarez, C., Garces-Zarzalejo, C., Alonso-Aguirre, M. A., Salas-Venero, C., Martínez-Martínez, L., and Fariñas, M. C.: Accuracy of different diagnostic tests for early, delayed and late prosthetic joint infection, BMC Infectious Diseases, 17, 592, https://doi.org/10.1186/s12879-017-2693-1, 2017.
Fernandez-Sampedro, M., Sanlés-González, I., García-Ibarbia, C., Fañanás-Rodríquez, N., Fakkas-Fernández, M., and Fariñas, M. C.: The poor accuracy of D-dimer for the diagnosis of prosthetic joint infection but its potential usefulness in early postoperative infections following revision arthroplasty for aseptic loosening, BMC Infectious Diseases, 22, 91, https://doi.org/10.1186/s12879-022-07060-8, 2022.
Fink, B., Makowiak, C., Fuerst, M., Berger, I., Schäfer, P., and Frommelt, L.: The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements, The Journal of Bone and Joint Surgery, 90, 874–878, https://doi.org/10.1302/0301-620x.90b7.20417, 2008.
Fink, B., Gebhard, A., Fuerst, M., Berger, I., and Schäfer, P.: High diagnostic value of synovial biopsy in periprosthetic joint infection of the hip, Clinical Orthopaedics and Related Research, 471, 956–964, https://doi.org/10.1007/s11999-012-2474-5, 2013.
Fink, B., Steurer, M., Hofäcker, S., Schäfer, P., Sandow, D., Schuster, P., and Oremek, D.: Preoperative PCR analysis of synovial fluid has limited value for the diagnosis of periprosthetic joint infections of total knee arthroplasties, Archives of orthopaedic and traumatic surgery, Archiv fur orthopadische und Unfall-Chirurgie, 138, 871–878, https://doi.org/10.1007/s00402-018-2924-y, 2018.
Fink, B., Schlumberger, M., Beyersdorff, J., and Schuster, P.: C-reactive protein is not a screening tool for late periprosthetic joint infection, Journal of orthopaedics and traumatology: official journal of the Italian Society of Orthopaedics and Traumatology, 21, 2, https://doi.org/10.1186/s10195-020-0542-2, 2020.
Fu, J., Ni, M., Chai, W., Li, X., Hao, L., and Chen, J.: Synovial Fluid Viscosity Test is Promising for the Diagnosis of Periprosthetic Joint Infection, The Journal of Arthroplasty, 34, 1197–1200, https://doi.org/10.1016/j.arth.2019.02.009, 2019.
Gallo, J., Svoboda, M., Zapletalova, J., Proskova, J., and Juranova, J.: Serum IL-6 in combination with synovial IL-6/CRP shows excellent diagnostic power to detect hip and knee prosthetic joint infection, PloS one, 13, e0199226, https://doi.org/10.1371/journal.pone.0199226, 2018.
Ghanem, E., Antoci, V., Jr., Pulido, L., Joshi, A., Hozack, W., and Parvizi, J.: The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty, International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases, 13, e444–e449, https://doi.org/10.1016/j.ijid.2009.02.017, 2009.
Gollwitzer, H., Dombrowski, Y., Prodinger, P. M., Peric, M., Summer, B., Hapfelmeier, A., Saldamli, B., Pankow, F., von Eisenhart-Rothe, R., Imhoff, A. B., Schauber, J., Thomas, P., Burgkart, R., and Banke, I. J.: Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection, The Journal of Bone and Joint Surgery, 95, 644–651, https://doi.org/10.2106/jbjs.L.00205, 2013.
Greidanus, N. V., Masri, B. A., Garbuz, D. S., Wilson, S. D., McAlinden, M. G., Xu, M., and Duncan, C. P.: Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation, The Journal of Bone and Joint Surgery, 89, 1409–1416, https://doi.org/10.2106/jbjs.D.02602, 2007.
Grzelecki, D., Walczak, P., Grajek, A., Szostek, M., Dudek, P., Bartosz, P., Olewnik, Ł., Czubak-Wrzosek, M., Marczak, D., and Tyrakowski, M.: Elevated plasma D-dimer concentration has higher efficacy for the diagnosis of periprosthetic joint infection of the knee than of the hip-A single-center, retrospective study, Journal of Orthopaedic Research: official publication of the Orthopaedic Research Society, 39, 291–298, https://doi.org/10.1002/jor.24897, 2021.
Higgins, J. P. and Thompson, S. G.: Quantifying heterogeneity in a meta-analysis, Stat. Med., 21, 1539–1558, https://doi.org/10.1002/sim.1186, 2002.
Hu, Q., Fu, Y., and Tang, L.: Serum D-dimer as a diagnostic index of PJI and retrospective analysis of etiology in patients with PJI, Clin. Chim. Acta, 506, 67–71, https://doi.org/10.1016/j.cca.2020.03.023, 2020.
Huang, J., Wang, J., Qin, L., Zhu, B., Huang, W., and Hu, N.: Combination of Synovial Fluid IL-4 and Polymorphonuclear Cell Percentage Improves the Diagnostic Accuracy of Chronic Periprosthetic Joint Infection, Frontiers in Surgery, 9, 843187, https://doi.org/10.3389/fsurg.2022.843187, 2022.
Itasaka, T., Kawai, A., Sato, T., Mitani, S., and Inoue, H.: Diagnosis of infection after total hip arthroplasty, Journal of Orthopaedic Dcience: Official Journal of the Japanese Orthopaedic Association, 6, 320–326, https://doi.org/10.1007/s007760100026, 2001.
Klemt, C., Tirumala, V., Smith, E. J., Xiong, L., and Kwon, Y. M.: Complete blood platelet and lymphocyte ratios increase diagnostic accuracy of periprosthetic joint infection following total hip arthroplasty, Archives of orthopaedic and traumatic surgery, Archiv fur orthopadische und Unfall-Chirurgie, 143, 1441–1449, https://doi.org/10.1007/s00402-021-04309-w, 2023.
Klim, S. M., Prattes, J., Amerstorfer, F., Niedrist, T., Zurl, C., Stradner, M., Dreo, B., Glehr, G., Leithner, A., Glehr, M., Reinbacher, P., Sadoghi, P., and Hauer, G.: Soluble Urokinase Plasminogen Activator Receptor (SuPAR) Analysis for Diagnosis of Periprosthetic Joint Infection, Antibiotics (Basel, Switzerland), 13, https://doi.org/10.3390/antibiotics13020179, 2024.
Kuo, F. C., Lu, Y. D., Wu, C. T., You, H. L., Lee, G. B., and Lee, M. S.: Comparison of molecular diagnosis with serum markers and synovial fluid analysis in patients with prosthetic joint infection, The Bone & Joint Journal, 100-b, 1345–1351, https://doi.org/10.1302/0301-620x.100b10.bjj-2018-0096.r1, 2018.
Kuo, F. C., Lin, P. C., Yen, S. H., Tan, T. L., Wu, C. T., and Wang, J. W.: Which Minor Criteria is the Most Accurate Predictor for the Diagnosis of Hip and Knee Periprosthetic Joint Infection in the Asian Population?, The Journal of Arthroplasty, 37, 2076–2081, https://doi.org/10.1016/j.arth.2022.05.002, 2022.
Levent, A., Neufeld, M. E., Piakong, P., Lausmann, C., Gehrke, T., and Citak, M.: Which International Consensus Meeting Preoperative Minor Criteria is the Most Accurate Marker for the Diagnosis of Periprosthetic Joint Infection in Hip and Knee Arthroplasty?, The Journal of Arthroplasty, 36, 3728–3733, https://doi.org/10.1016/j.arth.2021.06.030, 2021.
Li, R., Shao, H. Y., Hao, L. B., Yu, B. Z., Qu, P. F., Zhou, Y. X., and Chen, J. Y.: Plasma Fibrinogen Exhibits Better Performance Than Plasma D-Dimer in the Diagnosis of Periprosthetic Joint Infection: A Multicenter Retrospective Study, The Journal of Bone and Joint Surgery, 101, 613–619, https://doi.org/10.2106/jbjs.18.00624, 2019.
Liu, H., Yu, Y., and Niu, Y.: Utility of Human Neutrophil Lipocalin as a Diagnosing Biomarker of Prosthetic Joint Infection: A Clinical Pilot Study, Infection and Drug Resistance, 15, 2393–2400, https://doi.org/10.2147/IDR.S355180, 2022.
Liu, J. Z., Saleh, A., Klika, A. K., Barsoum, W. K., and Higuera, C. A.: Serum Inflammatory Markers for Periprosthetic Knee Infection in Obese Versus Non-Obese Patients, Journal of Arthroplasty, 29, 1880–1883, https://doi.org/10.1016/j.arth.2014.07.005, 2014.
Maimaiti, Z., Xu, C., Fu, J., Chai, W., Zhou, Y., and Chen, J.: The Potential Value of Monocyte to Lymphocyte Ratio, Platelet to Mean Platelet Volume Ratio in the Diagnosis of Periprosthetic Joint Infections, Orthop. Surg., 14, 306–314, https://doi.org/10.1111/os.12992, 2022.
Majors, I. and Jagadale, V. S.: Serum interleukin 6 could be a valuable initial diagnostic tool in prosthetic knee joint infections, European Journal of Orthopaedic Surgery & Traumatology: Orthopedie Traumatologie, 29, 1781–1788, https://doi.org/10.1007/s00590-019-02519-y, 2019.
McNally, M., Sousa, R., Wouthuyzen-Bakker, M., Chen, A. F., Soriano, A., Vogely, H. C., Clauss, M., Higuera, C. A., and Trebše, R.: The EBJIS definition of periprosthetic joint infection, The Bone & Joint Journal, 103-b, 18–25, https://doi.org/10.1302/0301-620x.103b1.Bjj-2020-1381.R1, 2021.
Muñoz-Mahamud, E., Tornero, E., Estrada, J. A., Fernández-Valencia, J. A., Martínez-Pastor, J. C., and Soriano, Á.: Usefulness of serum D-dimer and platelet count to mean platelet volume ratio to rule out chronic periprosthetic joint infection, Journal of Bone and Joint Infection, 7, 109–115, https://doi.org/10.5194/jbji-7-109-2022, 2022.
Nilsdotter-Augustinsson, A., Briheim, G., Herder, A., Ljunghusen, O., Wahlström, O., and Ohman, L.: Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening, Acta Orthopaedica, 78, 629–639, https://doi.org/10.1080/17453670710014329, 2007.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., and Wilson, W. R.: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the infectious diseases Society of America, Clinical Infectious Diseases, 56, e1–e25, https://doi.org/10.1093/cid/cis803, 2013.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., McGuinness, L. A., Stewart, L. A., Thomas, J., Tricco, A. C., Welch, V. A., Whiting, P., and Moher, D.: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ, 372, 71, https://doi.org/10.1136/bmj.n71, 2021.
Pannu, T. S., Villa, J. M., Patel, P. D., Riesgo, A. M., Barsoum, W. K., and Higuera, C. A.: The Utility of Serum d-Dimer for the Diagnosis of Periprosthetic Joint Infection in Revision Total Hip and Knee Arthroplasty, The Journal of Arthroplasty, 35, 1692–1695, https://doi.org/10.1016/j.arth.2020.01.034, 2020.
Parvizi, J. and Gehrke, T.: Definition of periprosthetic joint infection, The Journal of Arthroplasty, 29, 1331, https://doi.org/10.1016/j.arth.2014.03.009, 2014.
Parvizi, J., Jacovides, C., Zmistowski, B., and Jung, K. A.: Definition of periprosthetic joint infection: is there a consensus?, Clinical Orthopaedics and Related Research, 469, 3022–3030, https://doi.org/10.1007/s11999-011-1971-2, 2011.
Parvizi, J., Jacovides, C., Adeli, B., Jung, K. A., and Hozack, W. J.: Mark B. Coventry Award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection, Clinical Orthopaedics and Related Research, 470, 54–60, https://doi.org/10.1007/s11999-011-1991-y, 2012.
Paziuk, T., Rondon, A. J., Goswami, K., Tan, T. L., and Parvizi, J.: A Novel Adjunct Indicator of Periprosthetic Joint Infection: Platelet Count and Mean Platelet Volume, The Journal of Arthroplasty, 35, 836–839, https://doi.org/10.1016/j.arth.2019.10.012, 2020.
Piper, K. E., Fernandez-Sampedro, M., Steckelberg, K. E., Mandrekar, J. N., Karau, M. J., Steckelberg, J. M., Berbari, E. F., Osmon, D. R., Hanssen, A. D., Lewallen, D. G., Cofield, R. H., Sperling, J. W., Sanchez-Sotelo, J., Huddleston, P. M., Dekutoski, M. B., Yaszemski, M., Currier, B., and Patel, R.: C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection, PloS one, 5, e9358, https://doi.org/10.1371/journal.pone.0009358, 2010.
Qin, L., Li, F., Gong, X., Wang, J., Huang, W., and Hu, N.: Combined Measurement of D-Dimer and C-Reactive Protein Levels: Highly Accurate for Diagnosing Chronic Periprosthetic Joint Infection, The Journal of Arthroplasty, 35, 229–234, https://doi.org/10.1016/j.arth.2019.08.012, 2020a.
Qin, L., Li, X., Wang, J., Gong, X., Hu, N., and Huang, W.: Improved diagnosis of chronic hip and knee prosthetic joint infection using combined serum and synovial IL-6 tests, Bone & Joint Research, 9, 587–592, https://doi.org/10.1302/2046-3758.99.Bjr-2020-0095.R1, 2020b.
Schinsky, M. F., Della Valle, C. J., Sporer, S. M., and Paprosky, W. G.: Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty, The Journal of Bone and Joint Surgery, 90, 1869–1875, https://doi.org/10.2106/jbjs.G.01255, 2008.
Shah, R. P., Plummer, D. R., Moric, M., Sporer, S. M., Levine, B. R., and Della Valle, C. J.: Diagnosing Infection in the Setting of Periprosthetic Fractures, The Journal of Arthroplasty, 31, 140–143, https://doi.org/10.1016/j.arth.2015.08.045, 2016.
Shahi, A., Kheir, M. M., Tarabichi, M., Hosseinzadeh, H. R. S., Tan, T. L., and Parvizi, J.: Serum D-Dimer Test Is Promising for the Diagnosis of Periprosthetic Joint Infection and Timing of Reimplantation, The Journal of Bone and Joint Surgery, 99, 1419–1427, https://doi.org/10.2106/jbjs.16.01395, 2017.
Shang, G., Fei, Z., Xu, H., Wang, Y., and Xiang, S.: Globulin and albumin to globulin ratio precisely diagnose periprosthetic joint infection and determine the timing of second-stage reimplantation, J. Orthop. Surg. Res., 17, 12, https://doi.org/10.1186/s13018-021-02899-0, 2022.
Shi, W., Jiang, Y., Tian, H., Wang, Y., Zhang, Y., Yu, T., and Li, T.: C-Reactive Protein-to-Albumin Ratio (CAR) and C-Reactive Protein-to-Lymphocyte Ratio (CLR) are Valuable Inflammatory Biomarker Combination for the Accurate Prediction of Periprosthetic Joint Infection, Infection and Drug Resistance, 16, 477–486, https://doi.org/10.2147/idr.S398958, 2023.
Shohat, N., Bauer, T., Buttaro, M., Budhiparama, N., Cashman, J., Della Valle, C. J., Drago, L., Gehrke, T., Marcelino Gomes, L. S., Goswami, K., Hailer, N. P., Han, S. B., Higuera, C. A., Inaba, Y., Jenny, J. Y., Kjaersgaard-Andersen, P., Lee, M., Llinás, A., Malizos, K., Mont, M. A., Jones, R. M., Parvizi, J., Peel, T., Rivero-Boschert, S., Segreti, J., Soriano, A., Sousa, R., Spangehl, M., Tan, T. L., Tikhilov, R., Tuncay, I., Winkler, H., Witso, E., Wouthuyzen-Bakker, M., Young, S., Zhang, X., Zhou, Y., and Zimmerli, W.: Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? Can the Same Criteria be Used for Both Joints?: Proceedings of International Consensus on Orthopedic Infections, The Journal of Arthroplasty, 34, S325–s327, https://doi.org/10.1016/j.arth.2018.09.045, 2019.
Sigmund, I. K., Holinka, J., Staats, K., Sevelda, F., Lass, R., Kubista, B., Giurea, A., and Windhager, R.: Inferior performance of established and novel serum inflammatory markers in diagnosing periprosthetic joint infections, International Orthopaedics, 45, 837–846, https://doi.org/10.1007/s00264-020-04889-z, 2021.
Tetreault, M. W., Wetters, N. G., Moric, M., Gross, C. E., and Della Valle, C. J.: Is synovial C-reactive protein a useful marker for periprosthetic joint infection?, Clinical Orthopaedics and Related Research, 472, 3997–4003, https://doi.org/10.1007/s11999-014-3828-y, 2014.
Tirumala, V., Klemt, C., Xiong, L., Chen, W., van den Kieboom, J., and Kwon, Y. M.: Diagnostic Utility of Platelet Count/Lymphocyte Count Ratio and Platelet Count/Mean Platelet Volume Ratio in Periprosthetic Joint Infection Following Total Knee Arthroplasty, The Journal of Arthroplasty, 36, 291–297, https://doi.org/10.1016/j.arth.2020.07.038, 2021.
Tohtz, S. W., Muller, M., Morawietz, L., Winkler, T., and Perka, C.: Validity of frozen sections for analysis of periprosthetic loosening membranes, Clinical Orthopaedics and Related Research, 468, 762–768, https://doi.org/10.1007/s11999-009-1102-5, 2010.
Toossi, N., Adeli, B., Rasouli, M. R., Huang, R., and Parvizi, J.: Serum white blood cell count and differential do not have a role in the diagnosis of periprosthetic joint infection, The Journal of Arthroplasty, 27, 51–54, https://doi.org/10.1016/j.arth.2012.03.021, 2012.
Villacis, D., Merriman, J. A., Yalamanchili, R., Omid, R., Itamura, J., and Hatch, G. F. R.: Serum interleukin-6 as a marker of periprosthetic shoulder infection, Journal of Bone and Joint Surgery, 96, 41–45, https://doi.org/10.2106/JBJS.L.01634, 2014.
Wang, H., Qin, L., Wang, J., and Huang, W.: Synovial fluid IL-1β appears useful for the diagnosis of chronic periprosthetic joint infection, J. Orthop. Surg. Res., 16, 144, https://doi.org/10.1186/s13018-021-02296-7, 2021.
Wang, R., Shi, G., Zhang, H., Wang, T., Ren, W., and Jiao, Q.: Globulin and Albumin/Globulin Ratios as Potential Biomarkers for the Diagnosis of Acute and Chronic Peri-Prosthetic Joint Infections: A Retrospective Study, Surgical Infections, 24, 58–65, https://doi.org/10.1089/sur.2022.215, 2023a.
Wang, X., Zheng, Z., Wang, J., Ma, H., Wang, G., and Zhao, X.: Can Platelets/Mean Platelet Volume Accurately Diagnose Periprosthetic Joint Infection? Revealing Their Actual Diagnostic Efficacy, Infection and Drug Resistance, 16, 7155–7163, https://doi.org/10.2147/idr.S420323, 2023b.
Wang, Y., Li, Y., Qiao, L., and Sun, S.: Comparison of a Comprehensive Set of Fibrinolytic Markers With C-Reactive Protein and Erythrocyte Sedimentation Rate for the Diagnosis of Periprosthetic Joint Infection, The Journal of Arthroplasty, 35, 2613–2618, https://doi.org/10.1016/j.arth.2020.04.096, 2020.
Whiting, P. F., Rutjes, A. W., Westwood, M. E., Mallett, S., Deeks, J. J., Reitsma, J. B., Leeflang, M. M., Sterne, J. A., and Bossuyt, P. M.: QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies, Ann. Intern. Med., 155, 529–536, https://doi.org/10.7326/0003-4819-155-8-201110180-00009, 2011.
Worthington, T., Dunlop, D., Casey, A., Lambert, R., Luscombe, J., and Elliott, T.: Serum procalcitonin, interleukin-6, soluble intercellular adhesin molecule-1 and IgG to short-chain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision, British Journal of Biomedical Science, 67, 71–76, https://doi.org/10.1080/09674845.2010.11730294, 2010.
Wouthuyzen-Bakker, M., Ploegmakers, J. J. W., Ottink, K., Kampinga, G. A., Wagenmakers-Huizenga, L., Jutte, P. C., and Kobold, A. C. M.: Synovial Calprotectin: An Inexpensive Biomarker to Exclude a Chronic Prosthetic Joint Infection, The Journal of Arthroplasty, 33, 1149–1153, https://doi.org/10.1016/j.arth.2017.11.006, 2018.
Wu, C., Qu, X., Mao, Y., Li, H., Dai, K., Liu, F., and Zhu, Z.: Utility of intraoperative frozen section in the diagnosis of periprosthetic joint infection, PloS one, 9, e102346, https://doi.org/10.1371/journal.pone.0102346, 2014.
Wu, H., Meng, Z., Pan, L., Liu, H., Yang, X., and Yongping, C.: Plasma Fibrinogen Performs Better Than Plasma d-Dimer and Fibrin Degradation Product in the Diagnosis of Periprosthetic Joint Infection and Determination of Reimplantation Timing, The Journal of Arthroplasty, 35, 2230–2236, https://doi.org/10.1016/j.arth.2020.03.055, 2020.
Wu, Y., Sun, K., Liu, R., Wu, L., Zeng, Y., Li, M., Xu, J., and Shen, B.: C-reactive protein/albumin and C-reactive protein/fibrinogen ratios for the diagnosis of periprosthetic joint infection in revision total joint arthroplasty, International Immunopharmacology, 115, 109682, https://doi.org/10.1016/j.intimp.2023.109682, 2023.
Xu, H., Xie, J., Huang, Q., Lei, Y., Zhang, S., and Pei, F.: Plasma Fibrin Degradation Product and D-Dimer Are of Limited Value for Diagnosing Periprosthetic Joint Infection, The Journal of Arthroplasty, 34, 2454–2460, https://doi.org/10.1016/j.arth.2019.05.009, 2019.
Xu, H., Xie, J., Yang, J., Chen, G., Huang, Q., and Pei, F.: Plasma Fibrinogen and Platelet Count Are Referable Tools for Diagnosing Periprosthetic Joint Infection: A Single-Center Retrospective Cohort Study, The Journal of Arthroplasty, 35, 1361–1367, https://doi.org/10.1016/j.arth.2019.12.015, 2020.
Xu, H., Xie, J., Wang, D., Huang, Q., Huang, Z., and Zhou, Z.: Plasma levels of D-dimer and fibrin degradation product are unreliable for diagnosing periprosthetic joint infection in patients undergoing re-revision arthroplasty, J. Orthop. Surg. Res., 16, 628, https://doi.org/10.1186/s13018-021-02764-0, 2021.
Xu, H., Xie, J., Zhang, S., Wang, D., Huang, Z., and Zhou, Z.: Potential Blood Biomarkers for Diagnosing Periprosthetic Joint Infection: A Single-Center, Retrospective Study, Antibiotics (Basel, Switzerland), 11, https://doi.org/10.3390/antibiotics11040505, 2022.
Yang, F., Zhao, C., Huang, R., Ma, H., Wang, X., Wang, G., and Zhao, X.: Plasma fibrinogen in the diagnosis of periprosthetic joint infection, Scientific Reports, 11, 677, https://doi.org/10.1038/s41598-020-80547-z, 2021.
Ye, Y., Chen, W., Gu, M., Liu, Q., Xian, G., Pan, B., Zheng, L., Chen, X., Zhang, Z., and Sheng, P.: Limited value of serum neutrophil-to-lymphocyte ratio in the diagnosis of chronic periprosthetic joint infection, Journal of orthopaedics and traumatology: official journal of the Italian Society of Orthopaedics and Traumatology, 22, 37, https://doi.org/10.1186/s10195-021-00599-3, 2021.
Yin, H., Xu, D., and Wang, D.: Diagnostic value of next-generation sequencing to detect periprosthetic joint infection, BMC Musculoskeletal Disorders, 22, 252, https://doi.org/10.1186/s12891-021-04116-9, 2021.
Yu, B. Z., Fu, J., Chai, W., Hao, L. B., and Chen, J. Y.: Neutrophil to lymphocyte ratio as a predictor for diagnosis of early Periprosthetic joint infection, BMC Musculoskeletal Disorders, 21, 706, https://doi.org/10.1186/s12891-020-03704-5, 2020.
Yu, B. Z., Li, R., Li, X., Chai, W., Zhou, Y. G., and Chen, J. Y.: The relationship of C-reactive protein/interleukin-6 concentrations between serum and synovial fluid in the diagnosis of periprosthetic joint infection, J. Orthop. Surg. Res., 16, 733, https://doi.org/10.1186/s13018-021-02880-x, 2021.