the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Efficacy of rezafungin in a case of Candida spondylodiskitis
Marin Lahouati
Claire Tinévez
Frédéric Gabriel
Fabien Xuereb
Maxime Lefranc
Frédéric-Antoine Dauchy
Rezafungin, which only requires weekly administration, is a potential candidate for difficult-to-treat infections that require long-term antimicrobial treatment, such as bone and joint infections. We report the first case of Candida glabrata spondylodiskitis successfully treated with 3 weeks of caspofungin followed by 10 weeks of rezafungin.
- Article
(890 KB) - Full-text XML
- BibTeX
- EndNote
Bone and joint infections (BJIs) due to fungal agents are infrequent and challenging to treat, requiring extended antifungal treatment (Gamaletsou et al., 2022). Rezafungin, a next-generation echinocandin derived from anidulafungin, has a similar spectrum to other echinocandins with respect to Candida spp. and Aspergillus spp. STRIVE and ReSTORE trials led to Food and Drug Administration (FDA) approval of rezafungin for the treatment of candidaemia and invasive candidiasis in adult patients who have limited or no alternative treatment options (Thompson et al., 2024). Unfortunately, to date, there are no data on the efficacy of rezafungin with respect to BJIs, as these infections were excluded from the phase-III clinical trials. However, rezafungin has an innovative pharmacokinetic property, with enhanced tissue penetration and an extended elimination half-life of 130 h (Sandison et al., 2017). This long elimination half-life allows for a weekly intravenous infusion administration mode. This combination of factors makes rezafungin a potential candidate for difficult-to-treat infections that require long-term antimicrobial treatment, such as BJIs or endocarditis, in the same way that dalbavancin or oritavancin is used for treating Staphylococcus spp. infections. Here, we present the case study of a severe fungal BJI successfully treated with rezafungin.
A 63-year-old man with a history of diabetes, hypertension, and benign prostatic hypertrophy had a renal lithiasis, which led to obstructive pyelonephritis treated with the placement of a double-J stent in the ureter and antibiotherapy. A total of 1 month later, the double-J stent was removed. A few days later, the patient began to experience fever associated with back pain. A spine magnetic resonance imaging (MRI) scan revealed L4–L5 spondylodiskitis (Fig. 1).
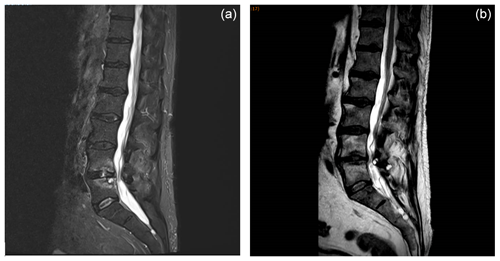
Figure 1MRI sagittal planes of L4–L5 spondylodiskitis showing (a) bone erosions and epiduritis at diagnosis and (b) the resolution of lesions 5 months after the end of rezafungin treatment.
The patient was hospitalized in the infectious diseases unit of Bordeaux University Hospital. Upon admission, the patient had no fever, severe lower-back pain, a C-reactive protein (CRP) value of 8.1 mg L−1, a leucocyte value of 7.2×109 L−1, and a slightly positive (7.6 pg mL−1 for a threshold of 7 pg mL−1) serum β-D-glucan (BDG) level (FUJIFILM Wako Chemicals Europe, Germany). Multiple L4–L5 radio-guided bone biopsies were performed, and all five biopsies yielded Candida glabrata (Nakaseomyces glabratus) in culture. Specific bacterial and mycobacterial cultures remained sterile. The C. glabrata strain exhibited cross-resistance to azole in vitro with a minimum inhibitory concentration (MIC) for fluconazole and voriconazole at 256 and 8 mg L−1, respectively. The MIC for caspofungin was 0.064 mg L−1, whereas it was 0.016 mg L−1 for micafungin; therefore, the isolate was presumed to be susceptible to rezafungin as well (Pfaller et al., 2016).
An intravenous treatment with caspofungin was carried out (70 mg d−1) for 3 weeks and was then followed by rezafungin (a first dose of 400 mg followed by a 200 mg dose weekly for 10 weeks). Rezafungin was well tolerated for the entire treatment period. Serum BDG was negative (<3 pg mL−1) after 2 weeks of caspofungin treatment. Due to clinical improvement and negative serum β-D-glucan results, we did not prolong antifungal treatment beyond 3 months. MRI examinations (performed 2 and 5 months after the conclusion of antifungal treatment) showed the regression of lesions (Fig. 1). At 5 months following the conclusion of antifungal treatment, the patient had gained weight and back pain had disappeared. The outcome was still favorable 3 months later (at the 8-month follow-up), and the patient will be monitored for a total duration of 2 years.
Cases of Candida spp. osteomyelitis are infrequent; thus, there are no randomized clinical trials to compare treatments for these BJIs. Gamaletsou et al. (2012) reported 207 cases of Candida spp. osteomyelitis, 51 % of which were located on vertebras. C. glabrata was only implicated in 17 cases (8 % of instances). The median treatment duration was 90 d, and treatment primarily involved an antifungal combination (45 % of instances), including amphotericin B or azoles. Azoles are often the only oral antifungal treatment available. However, approximately 30 % of the C. glabrata strains isolated in France are resistant to fluconazole (Desnos-Ollivier et al., 2021). In addition, azoles are known to have drug interactions that may limit their use, especially in frail, elderly patients receiving polypharmacotherapy. Thus, alternative options are poor. Amphotericin B may be an alternative, but there is a high risk of adverse events, such as nephrotoxicity, leading to early treatment discontinuation and promoting relapse. Caspofungin, micafungin, or anidulafungin may also be considered as alternatives (Gamaletsou et al., 2022); however, daily intravenous administration can lead to catheter-associated complications and decrease patients' quality of life. In these difficult-to-treat infections, rezafungin seems to be a promising therapeutic option. The higher and longer exposures achieved with rezafungin in vivo should have important benefits with respect to the prevention of biofilm-associated nosocomial infections (e.g., catheter-related infections) and reduce the length of hospital stays. Viceconte et al. (2024) reported a case in which C. parapsilosis spondylodiskitis was successfully treated by 26 weeks of rezafungin. It is of note that rezafungin was used after 10 weeks of effective antifungal treatment (anidulafungin, voriconazole, and liposomal amphotericin B), making the intrinsic effectiveness of rezafungin difficult to extrapolate.
Here, we report case in which C. glabrata spondylodiskitis was successfully treated by 3 weeks of caspofungin followed by 10 weeks of rezafungin. Larger series of case studies and larger cohorts are required to evaluate the real-life efficacy of rezafungin.
The data set is available from the corresponding author upon reasonable request.
MLa, CT, FX, and FD were involved in the conception of the study. MLe, FG, and CT provided the initial data set. MLa and CT performed the acquisition and analysis of data. All authors contributed to the draft and revisions and approved the final manuscript.
Marin Lahouati received support from Mundipharma to attend a medical conference. The other authors have no competing interests to declare.
Informed consent was obtained from the patient involved in the case study presented in this report.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This paper was edited by Parham Sendi and reviewed by three anonymous referees.
Desnos-Ollivier, M., Lortholary, O., Bretagne, S., and Dromer, F.: Azole Susceptibility Profiles of More than 9,000 Clinical Yeast Isolates Belonging to 40 Common and Rare Species, Antimicrob. Agents Chemother., 65, e02615-20, https://doi.org/10.1128/AAC.02615-20, 2021.
Gamaletsou, M. N., Kontoyiannis, D. P., Sipsas, N. V., Moriyama, B., Alexander, E., Roilides, E., Brause, B., and Walsh, T. J.: Candida Osteomyelitis: Analysis of 207 Pediatric and Adult Cases (1970–2011), Clin. Infect. Dis., 55, 1338–1351, https://doi.org/10.1093/cid/cis660, 2012.
Gamaletsou, M. N., Rammaert, B., Brause, B., Bueno, M. A., Dadwal, S. S., Henry, M. W., Katragkou, A., Kontoyiannis, D. P., McCarthy, M. W., Miller, A. O., Moriyama, B., Pana, Z. D., Petraitiene, R., Petraitis, V., Roilides, E., Sarkis, J.-P., Simitsopoulou, M., Sipsas, N. V., Taj-Aldeen, S. J., Zeller, V., Lortholary, O., and Walsh, T. J.: Osteoarticular Mycoses, Clin. Microbiol. Rev., 35, e00086-19, https://doi.org/10.1128/cmr.00086-19, 2022.
Pfaller, M. A., Messer, S. A., Rhomberg, P. R., Jones, R. N., and Castanheira, M.: Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates, J. Antimicrob. Chemother., 71, 2868–2873, https://doi.org/10.1093/jac/dkw214, 2016.
Sandison, T., Ong, V., Lee, J., and Thye, D.: Safety and Pharmacokinetics of CD101 IV, a Novel Echinocandin, in Healthy Adults, Antimicrob. Agents Chemother., 61, e01627-16, https://doi.org/10.1128/AAC.01627-16, 2017.
Thompson, G. R., Soriano, A., Honore, P. M., Bassetti, M., Cornely, O. A., Kollef, M., Kullberg, B. J., Pullman, J., Hites, M., Fortún, J., Horcajada, J. P., Kotanidou, A., Das, A. F., Sandison, T., Aram, J. A., Vazquez, J. A., and Pappas, P. G.: Efficacy and safety of rezafungin and caspofungin in candidaemia and invasive candidiasis: pooled data from two prospective randomised controlled trials, Lancet Infect. Dis., 24, 319–328, https://doi.org/10.1016/S1473-3099(23)00551-0, 2024.
Viceconte, G., Buonomo, A. R., Esposito, N., Cattaneo, L., Somma, T., Scirocco, M. M., Mainolfi, C. G., and Gentile, I.: Salvage Therapy with Rezafungin for Candida parapsilosis Spondylodiscitis: A Case Report from Expanded Access Program, Microorganisms, 12, 903, https://doi.org/10.3390/microorganisms12050903, 2024.




