the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Osteosynthesis-associated infection of the lower limbs by multidrug-resistant and extensively drug-resistant Gram-negative bacteria: a multicentre cohort study
Efthymia Giannitsioti
Mauro José Salles
Andreas Mavrogenis
Dolors Rodriguez-Pardo
Ibai Los-Arcos
Alba Ribera
Javier Ariza
María Dolores del Toro
Sophie Nguyen
Eric Senneville
Eric Bonnet
Monica Chan
Maria Bruna Pasticci
Sabine Petersdorf
Natividad Benito
Nuala O' Connell
Antonio Blanco García
Gábor Skaliczki
Pierre Tattevin
Zeliha Kocak Tufan
Nikolaos Pantazis
Panayiotis D. Megaloikonomos
Panayiotis Papagelopoulos
Alejandro Soriano
Antonios Papadopoulos
Purpose: The purpose of this study was the clinical and therapeutic assessment of lower-limb osteosynthesis-associated infection (OAI) by multidrug-resistant (MDR) and extensively drug-resistant (XDR) Gram-negative bacteria (GNB), which have been poorly studied to date. Methods: A prospective multicentre observational study was conducted on behalf of ESGIAI (the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group on Implant-Associated Infections). Factors associated with remission of the infection were evaluated by multivariate and Cox regression analysis for a 24-month follow-up period. Results: Patients (n=57) had a history of trauma (87.7 %), tumour resection (7 %) and other bone lesions (5.3 %). Pathogens included Escherichia coli (n=16), Pseudomonas aeruginosa (n=14; XDR 50 %), Klebsiella spp. (n=7), Enterobacter spp. (n=9), Acinetobacter spp. (n=5), Proteus mirabilis (n=3), Serratia marcescens (n=2) and Stenotrophomonas maltophilia (n=1). The prevalence of ESBL (extended-spectrum β-lactamase), fluoroquinolone and carbapenem resistance were 71.9 %, 59.6 % and 17.5 % respectively. Most patients (n=37; 64.9 %) were treated with a combination including carbapenems (n=32) and colistin (n=11) for a mean of 63.3 d. Implant retention with debridement occurred in early OAI (66.7 %), whereas the infected device was removed in late OAI (70.4 %) (p=0.008). OAI remission was achieved in 29 cases (50.9 %). The type of surgery, antimicrobial resistance and duration of treatment did not significantly influence the outcome. Independent predictors of the failure to eradicate OAI were age >60 years (hazard ratio, HR, of 3.875; 95 % confidence interval, CI95 %, of 1.540–9.752; p=0.004) and multiple surgeries for OAI (HR of 2.822; CI95 % of 1.144–6.963; p=0.024). Conclusions: Only half of the MDR/XDR GNB OAI cases treated by antimicrobials and surgery had a successful outcome. Advanced age and multiple surgeries hampered the eradication of OAI. Optimal therapeutic options remain a challenge.
- Article
(878 KB) - Full-text XML
-
Supplement
(544 KB) - BibTeX
- EndNote
Orthopaedic implantable devices have geometrically increased during the last decades due to technological and medical advancements that have allowed orthopaedic interventions which were not feasible in the past (Trampuz and Zimmerli, 2006). Osteosynthesis is mandatory to ensure the anatomical reconstruction of damaged bone, promoting fusion within time. However, osteosynthesis could be complicated by infection (1 %–30 %) (Trampuz and Zimmerli, 2006), especially in elderly or frail populations with open fractures. In turn, osteosynthesis-associated infections (OAIs) may impair bone healing due to long-standing inflammatory process with increased risk of functional loss or even amputation of the infected limb (Trampuz and Zimmerli, 2006; Lew and Waldvogel, 2004; Metsemakers et al., 2018). Moreover, remission of OAI can usually be achieved via removal of the infected device (Trampuz and Zimmerli, 2006; Metsemakers et al., 2018; Steinmetz et al., 2019). However, explantation of the infected implant could be complex, especially in frail patients; therefore, surgical debridement with implant retention can be a reliable therapeutic option (Tschudin-Sutter et al., 2016).
Although Gram-positive cocci are the most implicated pathogens in osteomyelitis (Uçkay et al., 2012), Gram-negative bacteria (GNB) emerge as causative agents in 10 %–20 % of cases in the form of mono- or polymicrobial osteoarticular infections (Benito et al., 2016). Case series (with or without implantable devices) have been reported (Kanellakopoulou et al., 2009; Legout et al., 2006; Titécat et al., 2013), but most cases refer to prosthetic joint infection (PJI) (Benito et al., 2016), Nowadays, the emergence of multidrug-resistant (MDR) or even extensively drug-resistant (XDR) GNB, especially among Enterobacterales, bears novel diagnostic and therapeutic challenges worldwide. In a case series of chronic osteomyelitis during last decade, the incidence of MDR GNB was shown to increase over the years (Koutserimpas et al., 2018), but data on orthopaedic implants are limited. In the era of antimicrobial resistance, treatment options for orthopaedic implant infections are sparse, as eradication of these biofilm-related infections is very difficult (Trampuz and Zimmerli, 2006; Tschudin-Sutter et al., 2016; Benito et al., 2016). For this purpose, a multinational cohort study on MDR/XDR GNB orthopaedic device infections was launched on behalf of the European Study Group of Implant-Associated Infections (ESGIAI). Analysis of the clinical characteristics, treatment options and outcomes of MDR/XDR GNB PJI have recently been published by our group (Papadopoulos et al., 2019). However, pathogenicity, clinical features and therapeutic strategies are different in PJI and in fracture-related infections (Metsemakers et al., 2018; Steinmetz et al., 2019). There are very few case reports and case series – often mixed with PJI – describing multiresistant GNB orthopaedic device infection (Jorge et al., 2017; Pfang et al., 2019; Krajewski et al., 2014; Odio et al., 2015; Ribera et al., 2015; Siebenbürger et al., 2019). Therefore, we aimed to analyse treatment options and predictors of outcome in a larger series of patients with non-PJI osteosynthesis-associated infection (OAI) of the lower limbs by MDR/XDR GNB.
2.1 Study design
We conducted a multicentre retrospective evaluation of prospectively collected data from 2005 to 2015 on OAI of the lower limbs caused by MDR and XDR GNB. The aim of the study was to identify factors associated with remission of OAI. A total of 18 European and overseas medical institutions participated in the study, which was approved by each local ethics committee.
2.2 Data collection
Data on patients' age and comorbidities, history of trauma, prior history of bone infection treated by orthopaedic surgery, prior use of antibiotics and clinical presentation of the current OAI episode were recorded. GNB isolates from tissue/bone/device or blood cultures and their resistance pattern (multidrug or extensively drug resistant) were identified. The surgical treatment applied to each patient for the current OAI event and the antimicrobial therapy (regimen, monotherapy vs. combination, length of intravenous (IV) administration and total duration of treatment) were assessed. Factors related to remission of the infection were evaluated at the 24-month follow-up. Data were recorded using a case report form (CRF), which was completed by investigators at each participating centre. The evaluation of collected data was performed by Antonios Papadopoulos and Efthymia Giannitsioti.
2.3 Definitions
The definitions used in this work are as follows:
-
Osteomyelitis was identified upon location of the infection (long and square bones) according to standard definitions and international consensus (Lew and Waldvogel, 2004; Uçkay et al., 2012). Diagnosis of osteomyelitis in the case of the fracture of peripheral bones and subsequent reconstitution surgery relied on positive intraoperative tissue/bone cultures and/or histology along with compatible imaging diagnosis (Glaudemans et al., 2019). Moreover, early infection was defined as the presence of OAI in the 4 weeks following the index surgery. Any OAI event that occurred later than 4 weeks post-surgery was defined as late infection.
-
GNB isolates were identified by routine conventional biochemical and metabolic tests or MALDI-ToF (matrix-assisted laser desorption ionization time-of-flight) mass spectrometry. Antimicrobial susceptibility was assessed at each participating centre according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations (http://www.eucast.org/clinical_breakpoint/, last access: 1 January 2022).
-
MDR was defined as non-susceptibility to at least one agent in three or more antimicrobial categories (aminoglycosides; tetracyclines; cephalosporins; carbapenems; fluoroquinolones; penicillins and β-lactamase inhibitors; monobactams; phosphonic acids; and polymyxins). XDR was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e. bacterial isolates remain susceptible to only one or two categories) (Magiorakos et al., 2012).
-
With respect to the evaluation of treatment, surgery for infection performed upon diagnosis of OAI of the lower limbs was defined as (i) removal of the infected implant (with or without exchange) or (ii) retention of the implant with debridement. An early surgical procedure was defined as operation within 4 weeks of OAI, whereas a late surgical procedure was a procedure carried out after 4 weeks. Antimicrobial treatment given post-surgically was analysed as either monotherapy by a sole agent or as combined antibiotic treatment (more than one concomitant agents). The duration of antimicrobial treatment was defined by a 6-week course of antibiotics.
-
Outcome was defined as remission of OAI if no clinical, laboratory or microbiological sign of infection was assessed during the 24-month post-treatment follow-up period. Any other condition was considered to be non-remission and treatment failure.
2.4 Statistical analysis
Descriptive statistics were given by type of pathogen and resistance (MDR or XDR) and type of surgical treatment (implant removal vs. implant retention with debridement). Mean values were expressed with standard deviations (SDs). Comparative analyses of the characteristics of the study population were carried out using two-sample t tests for continuous variables and chi-square tests (or Fisher's exact tests where necessary) for categorical variables. Probabilities of remission of the infection after surgical and medical therapy were modelled using univariate and multivariate logistic regression. The risk of non-remission during the follow-up period was analysed using Cox regression survival analysis for competing risks data. Taking the time to failure into consideration, Kaplan–Meier survival analysis was performed, and the log-rank (Mantel–Cox) statistic and related hazard ratio (HR) with a 95 % confidence interval (CI) were reported. p values less than 0.05 were considered to be statistically significant. All analyses were performed using SPSS (version 22.0, SPSS Inc., IBM).
A total of 57 patients with OAI were included in the study. Osteosynthesis was performed due to trauma (n=50; 87.7 %), tumour resection (n=4; 7 %) and other, non-specified bone lesions (n=3; 5.3 %). Early and late OAI were detected in 28 and 29 patients respectively. In late cases, the median (interquartile range, IQR) time from the index surgery to the surgery for OAI was 13 (8–40) months. The demographics, clinical characteristics and treatment of patients subdivided by the type of surgical treatment are presented in Table S1. Patients' mean age (SD) was 56.30 (21.65) years with a female predominance (n=34, 59.6 %). A total of 29.8 % of patients presented with comorbidities. A total of 19 patients (33.3 %) had undergone a previous surgery for orthopaedic infection. Osteosynthesis was instrumented at the area of the hip (n=15), femur (n=13), knee (n=3), tibia (n=19), ankle (n=2) and metatarsal bones (n=5). Soft tissue infection (59.6 %) and sinus tract (42.1 %) were common clinical signs, whereas fever was only present in 19.3 % of cases. Bone damage compatible with osteomyelitis was documented in all patients. Prior use of antibiotics was present in 65 % of patients.
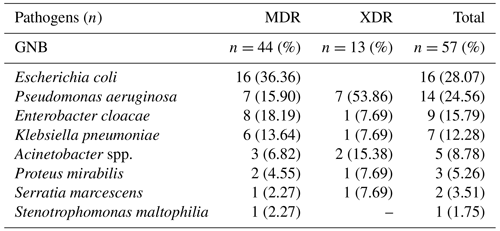
The distribution of pathogens as either MDR (n=44, 77.2 %) or XDR (n=13, 22.8 %) is depicted in Table 1. All Escherichia coli strains (n=16) were MDR, producing ESBL (extended-spectrum β-lactamases). XDR accounted for 50 % and 40 % of Pseudomonas aeruginosa and Acinetobacter spp. strains respectively. Overall, pathogens were identified as follows: ESBL-producing pathogens, which, by definition, are resistant to aztreonam (n=41; 71.9 %); fluoroquinolone-resistant pathogens (n=34; 59.6 %); aminoglycoside-resistant pathogens (n=24; 42.1 %); and carbapenem-resistant pathogens (n=10; 17.5 %). For GNB other than P. aeruginosa, resistance to co-trimoxazole and tigecycline reached 35 % and 7 % respectively. One XDR strain of P. aeruginosa was also resistant to fosfomycin. All GNB strains were sensitive to colistin except for Proteus mirabilis and Stenotrophomonas maltophilia due to intrinsic resistance.
Table 1Identification of Gram-negative bacteria (GNB) multidrug-resistant (MDR) and extensively drug-resistant (XDR) bacteria in osteosynthesis-associated infection of the lower limbs.
Various antimicrobial combinations were post-surgically administered in 64.9 % of the patients. Monotherapy was given to 35.1 % of patients. Overall, fluoroquinolones were administered in 8 cases (14 %), carbapenems in 32 cases (56.1 %) and colistin in 11 cases (19.3 %). The mean (SD) overall duration of antimicrobial therapy was 63.3 (38) d, whereas the mean (SD) duration of intravenous administration was 59.4 (44.5) d. The long duration of intravenous antibiotics in some cases reflects the lack of oral alternatives for the continuation of treatment after hospital discharge. The length of treatment as well as the number of administered antibiotics (monotherapy vs. combined therapy) did not significantly affect remission of OAI. (Table 2). The clinical features of OAI, antimicrobial resistance patterns, the type and duration of antimicrobial administration, and the outcome did not significantly differ between the implant removal and implant retention groups (Table S1 in the Supplement). However, early OAI was initially mostly managed by implant retention with debridement (66.7 %), whereas late OAI was primary managed by implant removal (with or without exchange) (70.4 %; p=0.008; Table S1). Among the 27 cases of implant removal, reimplantation was assessed in 6 cases, and debridement without insertion of a new orthopaedic device was assessed in 21 cases (including 1 case of amputation). Remission of infection was achieved in 2 of 6 cases in the reimplantation group vs. 14 of 21 cases in the no-device group (p=0.187).
Overall, criteria for remission of the infection were achieved only in 29 patients (50.9 %). Figure 1a illustrates the outcome of OAI split by early vs. late infection and type of surgery. Remission was achieved in 46.4 % of early infections (45 % with implant retention and 50 % with implant removal) and in 55.1 % of late infections (40 % with implant retention and 63.1 % with implant removal). The type of surgical intervention did not significantly affect the outcome within the early vs. late OAI groups (p=0.292; odds ratio (OR) of 1.902; CI95 % of 0.663–5.457; Fig. 1a). Remission was achieved in 54.5 % of MDR OAI cases (n=24) vs. 38.5 % of XDR OAI cases (n=5, p=0.105) (Table 1). Remission reached 50 % (MDR) vs. 25 % (XDR) in early OAI and 60 % (MDR) vs. 44.4 % (XDR) in late OAI cases. Figure 1b illustrates the outcome of OAI split by early and late infection in MDR and XDR cases: no statistical difference was assessed within groups (OR of 0.521; CI95 % of 0.147–1.846; p=0.358).
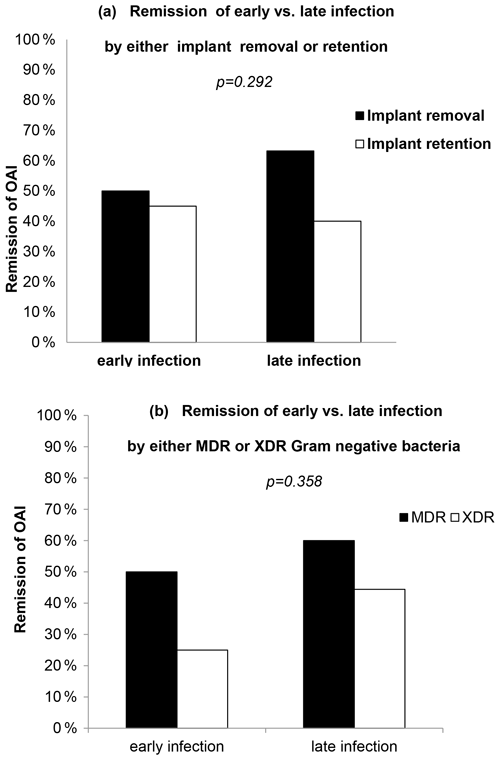
Figure 1Remission of OAI split by (a) type of surgical treatment or (b) type of antimicrobial resistance.
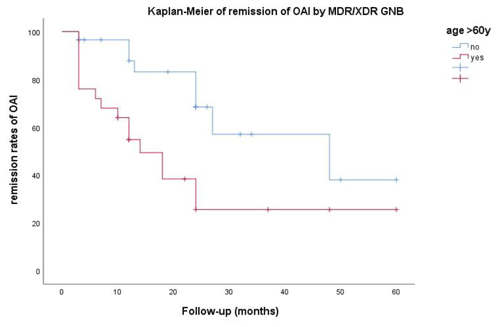
Univariate and adjusted multivariate logistic regression analysis demonstrated that the presence of comorbidities and multiple previous surgeries correlated with treatment outcome in patients aged >60 years. Although not statistically significant, failure of remission of OAI was more frequent for XDR than for MDR pathogens (61.5 % vs. 45.5 %; p=0.105) and in cases of extended antimicrobial treatment (more than 42 d (n=33) vs. less than 42 d (n=24); p=0.161) (Table 2). Figure 2 depicts the remission of OAI in patients split by age – more or less than 60 years old (log rank x2=7.919; 1 degree of freedom (DF); p=0.005). Factors associated with infection eradication at the 24-month follow-up were further analysed using the Cox regression model including age, MDR vs. XDR resistance, number of revision procedures for OAI and total duration of antimicrobial treatment. Only an age >60 years (HR of 3.875; CI95 % of 1.540–9.752; p=0.004) and multiple surgeries for OAI (HR of 2.822; CI95 % of 1.144–6.963; p=0.024) were independent predictors of failure (Table 3).
Table 2Univariate and multivariate logistic regression analysis of factors related to the remission of osteosynthesis-associated infections (OAIs) of the lower limbs by multidrug-resistant (MDR) and extensively drug-resistant (XDR) Gram-negative bacteria (GNB).
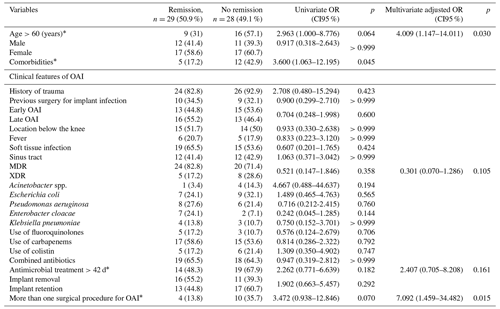
* Variables included in the multivariate model.
Table 3Multivariate Cox regression analysis of factors related to lack of eradication of osteosynthesis-associated infection (OAI) during a 24-month follow-up.
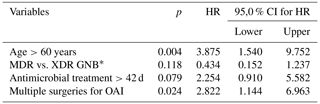
* The abbreviations used in the table are as follows: MDR – multidrug resistant, XDR – extensively drug resistant, and GNB – Gram-negative bacteria.
To our knowledge, this is the largest cohort study on lower-limb OAI by MDR/XDR GNB. Only a few case reports and very small case series on carbapenem-resistant Enterobacterales, P. aeruginosa and Acinetobacter spp. including both PJI and other OAI have been described (Jorge et al., 2017; Pfang et al., 2019; Krajewski et al., 2014; Odio et al., 2015; Ribera et al., 2015; Siebenbürger et al., 2019; Pasticci et al., 2014; Papagelopoulos et al., 2007). Globally, remission of OAI by GNB ranges from 27 % to 80 % (Martínez-Pastor et al., 2010; Grossi et al., 2016), reflecting a high variability among studies. OAI is characterized by the high prevalence of frailty of the host and by limited therapeutic options (Legout et al., 2006; Martinez-Pastor et al., 2010; Grossi et al., 2016; Rodriguez-Pardo et al., 2014). In the above-mentioned reports, most patients had received antibiotics prior to the current OAI event, as in our cohort (Benito et al., 2016; Pfang et al., 2019). The type of surgical intervention (removal vs. retention of the infected device) was not associated with remission. However, a recent study of 25 patients with osteoarticular infections (both PJI and non-PJI) by MDR/XDR GNB reported a cure rate of 100 % in patients treated by implant removal vs. only 33 % in patients treated with implant retention (Pfang et al., 2019). In our study, the overall infection eradication rate was lower even after removal of the infected implant. The type of antimicrobial resistance and the type of surgical intervention did not significantly affect the outcome, which is a finding that could be potentially be explained by the small number of patients. However, the fact that early XDR OAI – mostly treated by implant retention – presented a very low remission rate (25 %) highlights the need for radical surgery in cases of extended antimicrobial resistance. Moreover, in our previously published cohort of PJI by MDR/XDR GNB, higher remission rates of the infection (up to 80 %) were achieved with removal than with retention of the prosthesis (50 %) (Papadopoulos et al., 2019). Different patterns of treatment success between PJI and OAI may be due to the different nature and objectives of the orthopaedic devices (Metsemakers et al., 2018; Steinmetz et al., 2019). Removal of the infected implant is often postponed in OAI awaiting osseous healing and biomedical stability of the fractured bone. Therefore, the use of suppressive antibiotics can be applied until healing of the fracture is obtained, allowing the implant's removal. (Steinmetz et al., 2019; Tschudin-Sutter et al., 2016). Thus, remission of the infection can be hampered by slow osseous healing and the need for complex and repetitive operations, the initiation of embedded grafts, and revascularization (Trampuz and Zimmerli, 2006; Steinmetz et al., 2019). Under these circumstances, the optimal surgical and antimicrobial treatment is difficult to define, and the strategy of multiple operations in order to achieve healing and remission of the infection often fails. Our findings are in line with the study performed by Jorge et al. (2017) for fracture-related infections, which demonstrated that multiple surgeries, advanced age and MDR P. aeruginosa were predictors of treatment failure (Jorge et al., 2017). In our study, a trend towards a worse outcome for XDR compared with MDR infections was observed, but we failed to identify a significant difference due to the small sample size. Most patients were treated using a combination of antibiotics, as previously reported (Ribera et al., 2015; Brouqui et al., 1995; Rodriguez-Baño et al., 2018). For MDR and especially XDR cases with restricted antimicrobial treatment options, more experts recommend combined therapy tailored to drug susceptibility tests (Ribera et al., 2015; Rodriguez-Baño et al., 2018). Colistin is often the sole potent agent against the XDR GNB strains, including P. aeruginosa, K. pneumoniae and A. baumanii. The activity of colistin on biofilm formation has been proven in experimental studies; however, high doses of the drug concomitantly with other antibiotics are required (Lora-Tamayo et al., 2019). An in vivo experimental study demonstrated the clearance of a biofilm-related XDR GNB infection using a combination of colistin with fosfomycin or tigecycline, which are often the last treatment resort in XDR isolates (Corvec et al., 2013). Clinical data suggest the need for high-dose colistin for treatment effectiveness in osteoarticular infections by XDR P. aeruginosa (Valour et al., 2013). The combined systemic administration of colistin with its release by impregnated material has been proven to be successful (Krajewski et al., 2014; Papagelopoulos et al., 2007). However, the efficacy of IV colistin is significantly enhanced when combined with a β-lactam (Ribera et al., 2015). The low rates of remission of OAI in our study might be partly explained by the lack of fluoroquinolone administration due to high resistance rates. Ciprofloxacin has a proven penetration into the bone and promotes a successful outcome in GNB osteoarticular infections (Legout et al., 2006; Rodriguez-Pardo et al., 2014; Brouqui et al., 1995). Fluoroquinolones' resistance is one of the major drivers of the increased use of carbapenems (Papadopoulos et al., 2019; Martinez-Pastor et al., 2010). Novel therapeutic options for MDR/XDR GNB (like ceftazidime–avibactam and ceftolozane–tazobactam) were not available at the time of our study. As oral treatment was not available, most patients were treated using IV antibiotics for approximately 60 d, much longer than suggested (Lew and Waldvogel, 2004; Metsemakers et al., 2018; Uçkay et al., 2012). However, neither the combination of antibiotics nor a treatment duration of more than 42 d had a significant impact on the outcome, perhaps due to the sample size in our study. The eradication of infection was hampered by advanced age and the need for multiple surgical operations. Obviously, the immunological condition of the host, which is impaired with advanced age, may delay the ability of wound healing and bone reconstruction, as could poor vascularity and degenerative tissue structures. The lack of healthy tissue and tissue reconstitution capacity promotes persistent and relapsing infection (Steinmetz et al., 2019; Jamei et al., 2017).
This work has important limitations. It is an observational and retrospective cohort study. Therefore, the rates of antimicrobial resistance per institution are not available, as data for the study were collected through an invitation to the members of the ESGIAI study group, and a population-based study was not performed. The surgical management of cases of OAI is heterogenous, as a variety of surgical modalities and different techniques can be applied at different centres and in different countries. Moreover, the impact of local-delivery antibiotic carriage systems in OAI was not explored. Analysis of the genetic mechanisms of antimicrobial resistance in MDR or XDR GNB isolates was not available. However, this is the largest cohort of patients with OAI of the lower limbs by MDR/XDR GNB to date, with international recruitment from expert centres, providing a valuable insight on the characteristics and the outcomes of those particularly difficult to treat infections.
In conclusion, OAI of the lower limbs by MDR or XDR GNB was associated with low rates of remission at the 24-month follow-up. Only an age >60 years and multiple surgeries for OAI were linked to treatment failure. A continuous surveillance of emerging antimicrobial resistance and the efficacy of novel antimicrobial treatments along with the optimization of surgical interventions in fracture-related infections should be further explored via large prospective studies.
Patient data were collected using case report forms, which were completed by the author from each participating centre. The data are not publicly accessible, as they are provided by 19 European and overseas university and research institutions which are subject to different laws regarding the deposition of clinical data.
The supplement related to this article is available online at: https://doi.org/10.5194/jbji-7-279-2022-supplement.
Jaime Esteban-Moreno (Department of Clinical Microbiology, IIS-Fundación Jiménez Díaz, Madrid, Spain); Joaquín García Cañete (Bone and Joint Infection Unit, IIS-Fundación Jiménez Díaz, Madrid, Spain); Raúl Parrón (Bone and Joint Infection Unit, Department of Emergency Medicine, IIS-Fundación Jiménez Díaz Hospital, Madrid, Spain); David Lye (Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore); Rahmet Guner (Department of Infectious Diseases and Clinical Microbiology, Faculty of Medicine, Yildirim Beyazit University, Ankara Atatürk Education and Research Hospital, Ankara, Turkey); Laura Morata (Department of Infectious Diseases, Hospital Clínic, Barcelona, Spain); Ernesto Muñoz-Mahamud and Luis Lozano (Department of Orthopedics, Hospital Clínic, Barcelona, Spain); Carles Pigrau (Department of Infectious Diseases, Hospital Universitari Vall d'Hebron, Barcelona, Spain); Pablo S. Corona (Department of Orthopedic Surgery, Reconstructive Surgery and Septic Division, Hospital Universitari Vall d'Hebron, Barcelona, Spain); Maily Lung (Microbiology Department, Hospital Universitari Vall d'Hebron, Barcelona, Spain); Taiana Cunha Ribeiro and Giselle B. Klautau (Division of Infectious Diseases, Department of Internal Medicine, Santa Casa de São Paulo School of Medical Sciences, São Paulo, Brazil); Salvatore Cardaci (Infectious Diseases Unit, University of Perugia, Perugia, Italy); Yolanda Borrego Izquierdo (Infectious Diseases Unit, Hospital Universitario Virgen Macarena, Seville, Spain); Isabel Mur (Unit of Infectious Diseases, Department of Internal Medicine, Hospital de la Santa Creu i Sant Pau, Institut d'Investigació Biomèdica Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain); Xavier Crusi, Marcos Jordán and José Carlos González (Department of Orthopedics, Hospital de la Santa Creu i Sant Pau, Institut d'Investigació Biomèdica Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain); Alba Rivera (Department of Clinical Microbiology, Hospital de la Santa Creu i Sant Pau, Institut d'Investigació Biomèdica Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain); Marie Ghéno, Enora Ouamara-Digue, Anne Jolivet-Gougeon and Cédric Arvieux (Infectious Diseases and Intensive Care Unit, Pontchaillou University Hospital, Rennes, France); Gyula Prinz (Joined Saint Stephan and Saint Ladislaus Hospital, I Department of Internal Medicine, Budapest, Hungary); Botond Lakatos (Joined Saint Stephan and Saint Ladislaus Hospital, Department of Infectious Diseases, Budapest, Hungary); Nikolaos Antonakos, George Siakalis, Alice Dourou, Eleni Aggelou, Paraskevas Nikou and Sofia Athanasia (Fourth Department of Internal Medicine, University General Hospital Attikon, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece); Vasilios G. Igoumenou (First Department of Orthopaedics, University General Hospital Attikon, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece; Oscar Murillo and Joan Gómez-Junyent (Infectious Diseases Department, Hospital Universitari de Bellvitge, Barcelona); Jacier Cabo (Orthopaedic Surgery Department, Hospital Universitari de Bellvitge, Barcelona); Fe Tubau (Microbiology Department, Hospital Universitari de Bellvitge, Barcelona).
EG and AP conceived the idea and wrote the protocol. EG created the case report form. EG, MJS, AM, DRP, ILA, AR, JA, MDdT, SN, ES, EB, MC, MBP, SP, NB, NO'C, ABG, GS, PT, ZKT, NP, PDM, PP, AS and AP collected important data and completed the case report form. EG and AP analysed data. EG wrote the manuscript. NP provided statistical assistance. MJS, PT, AS, DRP, MDdT, AR, JA, NB, SP and ES provided significant comments on the first draft of the paper, and PT, AS and AP provided significant comments on the revised manuscript. The final version of the paper was approved by all authors.
At least one of the (co-)authors is a member of the editorial board of Journal of Bone and Joint Infection. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
This study was approved by each of the participating centres according to the rules of the local ethics committees regarding non-interventional, epidemiological, anonymously collected data.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was edited by Irene Karharina Sigmund and reviewed by two anonymous referees.
Benito, N., Franco, M., Ribera, A., Soriano, A., Rodriguez-Pardo, D., Sorlí, L., Fresco, G., Fernández-Sampedro, M., Dolores Del Toro, M., Guío, L., Sánchez-Rivas, E., Bahamonde, A., Riera, M., Esteban, J., Baraia-Etxaburu, J. M., Martínez-Alvarez, J., Jover-Sáenz, A., Dueñas, C., Ramos, A., Sobrino, B., Euba, G., Morata, L., Pigrau, C., Coll, P., Mur, I., Ariza, J., and REIPI (Spanish Network for Research in Infectious Disease) Group for the Study of Prosthetic Joint Infections: Time trends in the aetiology of prosthetic joint infections: a multicenter cohort study, Clin. Microbiol. Infect., 732, e1–732.e8, https://doi.org/10.1016/j.cmi.2016.05.004, 2016.
Brouqui, P., Rousseau, M. C., Stein, A., Drancourt, M., and Raoult, D.: Treatment of Pseudomonas aeruginosa-infected orthopedic prostheses with ceftazidime ciprofloxacin antibiotic combination, Antimicrob. Agents Chemother., 39, 2423–2425, https://doi.org/10.1128/AAC.39.11.2423, 1995.
Corvec, S., Furustrand Tafin, U., Betrisey, B., Borens, O., and Trampuz, A.: Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model, Antimicrob. Agents Chemother., 57, 1421–1427, https://doi.org/10.1128/AAC.01718-12, 2013.
European Committee on Antimicrobial Susceptibility Testing (EUCAST): recommendations, http://www.eucast.org/clinical_breakpoint/, last access: 1 January 2022.
Glaudemans, A. W. J. M., Jutte, P. C., Cataldo, M. A., Cassar-Pullicino, V., Gheysens, O., Borens, O., Trampuz, A., Wörtler, K., Petrosillo, N., Winkler, H., Signore, A., and Sconfienza, L. M.: Consensus document for the diagnosis of peripheral bone infection in adults: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement), Eur. J. Nucl. Med. Molec. Imaging, 46, 957–970, https://doi.org/10.1007/s00259-019-4262-x, 2019.
Grossi, O., Asseray, N., Bourigault, C., Corvec, S., Valette, M., Navas, D., Happi-Djeukou, L., Touchais, S., Bémer, P., Boutoille, D., and Nantes Bone and Joint Infections Study Group: Gram-negative prosthetic joint infections managed according to a multidisciplinary standardized approach: risk factors for failure and outcome with and without fluoroquinolones, J. Antimicrob. Chemother., 71, 2593–2597, https://doi.org/10.1093/jac/dkw202, 2016.
Jamei, O., Gjoni, S., Zenelaj, B., Kressmann, B., Belaieff, W., Hannouche, D., and Uçkay, I.: Which Orthopaedic Patients Are Infected with Gram-negative Non-fermenting Rods?, J. Bone Joint Infect., 2, 73–76, https://doi.org/10.7150/jbji.17171, 2017.
Jorge, L. G., Chueire, A. G., Fucuta, P. S., Machado, M. N., Oliveira, M. G., Nakazone, M. A., and Salles, M. J.: Predisposing factors for recurrence of chronic posttraumatic osteomyelitis: a retrospective observational cohort study from a tertiary referral center in Brazil, Patient Saf. Surg., 17, 11, https://doi.org/10.1186/s13037-017-0133-1, 2017.
Kanellakopoulou, K., Giannitsioti, E., Papadopoulos, A., Athanassia, S., Giamarellos-Bourboulis, E. J., and Giamarellou, H.: Chronic bone infections due to Enterobacter cloacae: current therapeutic trends and clinical outcome, J. Chemother., 21, 226–228, https://doi.org/10.1179/joc.2009.21.2.226, 2009.
Koutserimpas, C., Samonis, G., Plataki, M. N., Bikis, C., Kontakis, G., and Kofteridis, D. P.: Multidrug-resistant Gram-negative osteomyelitis: a 10-year study, G. Chir., 34, 284–290, 2018.
Krajewski, J., Bode-Böger, S. M., Tröger, U., Martens-Lobenhoffer, J., Mulrooney, T., Mittelstädt, H., Russlies, M., Kirchner, R., and Knobloch, K.-M. J.: Successful treatment of extensively drug-resistant Pseudomonas aeruginosa osteomyelitis using a colistin- and tobramycin-impregnated PMMA spacer, Int. J. Antimicrob. Agents, 44, 363–366, https://doi.org/10.1016/j.ijantimicag.2014.05.023, 2014.
Legout, L., Senneville, E., Stern, R., Yazdanpanah, Y., Savage, C., Roussel-Delvalez, H., Rosele, B., Migaud, H., and Mouton, Y.: Treatment of bone and joint infections caused by Gram-negative bacilli with cefepime-fluoroquinolone combination, Clin. Microbiol. Infect., 12, 1030–1033, https://doi.org/10.1111/j.1469-0691.2006.01523.x, 2006.
Lew, D. P. and Waldvogel, F. A.: Osteomyelitis, Lancet, 364, 369–379, https://doi.org/10.1016/S0140-6736(04)16727-5, 2004.
Lora-Tamayo, J., Murillo, O., and Ariza, J.: Clinical use of colistin in biofilm-associated infection, in: Polymyxin Antibiotics: From Laboratory Bench to Bedside, Advances in Experimental Medicine and Biology, edited by: Li, J. and Donelli, G., Springer, 1145, https://doi.org/10.1007/978-3-030-16373-0_13, 2019.
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., Harbarth, S., Hindler, J. F., Kahlmeter, G., Olsson-Liljequist, B., Paterson, D. L., Rice, L. B., Stelling, J., Struelens, M. J., Vatopoulos, A., Weber, J. T., and Monnet, D. L.: Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance, Clin. Microbiol. Infect., 18, 268–281, https://doi.org/10.1111/j.1469-0691.2011.03570.x, 2012.
Martínez-Pastor, J. C., Vilchez, F., Pitart, C., Sierra, J. M., and Soriano, A.: Antibiotic resistance in orthopaedic surgery: acute knee prosthetic joint infections due to extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, Eur. J. Clin. Microbiol. Infect. Dis., 29, 1039–1041, https://doi.org/10.1007/s10096-010-0950-y, 2010.
Metsemakers, W. J., Morgenstern, M., McNally, M. A., Moriarty, T. F., McFadyen, I., Scarborough, M., Athanasou, N. A., Ochsner, P. E., Kuehl, R., Raschke, M., Borens, O., Xie, Z., Velkes, S., Hungerer, S., Kates, S. L., Zalavras, C., Giannoudis, P. V., Richards, R. G., and Verhofstad, M. H.: Fracture-related infection: a consensus on definition from an international expert group, Injury, 49, 505–510, https://doi.org/10.1016/j.injury.2017.08.040, 2018.
Odio, C. D., van Duin, D., Cober, E., Teixeira-Johnson, L., Schmitt, S., and de Sanctis J.: Carbapenem-Resistant Klebsiella Pneumoniae Osteomyelitis and Soft Tissue Infections: A Descriptive Case Series, J. Infect. Dis. Ther., 3, 1, https://doi.org/10.4172/2332-0877.1000200, 2015.
Papadopoulos, A., Ribera, A., Mavrogenis, A. F., Rodriguez-Pardo, D., Bonnet, E., Salles, M. J., Del Toro, M. D., Nguyen, S., Blanco-García, A., Skaliczki, G., Soriano, A., Benito, N., Petersdorf, S., Pasticci, M. B., Tattevin, P., Kocak Tufan, Z., Chan, M., O'Connell, N., Pantazis, N., Kyprianou, A., Pigrau, C., Megaloikonomos, P. D., Senneville, E., Ariza, J., Papagelopoulos, P. J., and Giannitsioti, E.: ESCMID Study Group for Implant-Associated Infections (ESGIAI) Multidrug-resistant and extensively drug-resistant Gram-negative prosthetic joint infections: Role of surgery and impact of colistin administration, Int. J. Antimicrob. Agents, 53, 294–301, https://doi.org/10.1016/j.ijantimicag.2018.10.018, 2019.
Papagelopoulos, P. J., Mavrogenis, A. F., Giannitsioti, E., Kikilas, A., Kanellakopoulou, K., and Soucacos, P. N.: Management of a multidrug-resistant Pseudomonas aeruginosa infected total knee arthroplasty using colistin. A case report and review of the literature, J. Arthroplasty, 22, 457–462, https://doi.org/10.1016/j.arth.2006.05.006, 2007.
Pasticci, M. B., Di Filippo, P., Pasqualini, L., Mencacci, A., Pallotto, C., Malincarne, L., and Baldelii, F.: Tolerability and efficacy of long-term treatment with daptomycin, ceftazidime and colistin in a patient with a polymicrobial, multidrug-resistant prosthetic joint reinfection: a case report, J. Med. Case Rep., 8, 186, https://doi.org/10.1186/1752-1947-8-186, 2014.
Pfang, B. G., García-Cañete, J., García-Lasheras, J., Blanco, A., Auñón, Á., Parron-Cambero, R., Macías-Valcayo, A., and Esteban, J.: Infection by Multidrug Resistant Enterobacteriaceae, J. Clin. Med., 8, 220, https://doi.org/10.3390/jcm8020220, 2019.
Ribera, A., Benavent, E., Lora-Tamayo, J., Tubau, F., Pedrero, S., Cabo, X., Ariza, J., amd Murillo, O.: Osteoarticular infection caused by MDR Pseudomonas aeruginosa: the benefits of combination therapy with colistin plus β-lactams, J. Antimicrob. Chemother., 70, 3357–3365, https://doi.org/10.1093/jac/dkv281, 2015.
Rodríguez-Baño, J., Gutiérrez-Gutiérrez, B., Machuca, I., and Pascuala, A.: Treatment of Infections Caused by Extended-Spectrum-BetaLactamase- AmpC-, and Carbapenemase-Producing Enterobacteriaceae, Clin. Microbiol. Rev., 31, e00079-17, https://doi.org/10.1128/CMR.00079-17, 2018.
Rodríguez-Pardo, D., Pigrau, C., Lora-Tamayo, J., Soriano, A., del Toro, M. D., Cobo, J., Palomino, J., Euba, G., Riera, M., Sánchez-Somolinos, M., Benito, N., Fernández-Sampedro, M., Sorli, L., Guio, L., Iribarren, J. A., Baraia-Etxaburu, J. M., Ramos, A., Bahamonde, A., Flores-Sánchez, X., Corona, P. S., Ariza, J., and REIPI Group for the Study of Prosthetic Infection: Gram-negative prosthetic joint infection: outcome of a debridement, antibiotics and implant retention approach. A large multicentre study, Clin. Microbiol. Infect., 20, O911–919, https://doi.org/10.1111/1469-0691.12649, 2014.
Siebenbürger, G., Grabein, B., Schenck, T., Kammerlander, C., Böcker, W., and Zeckey, C.: Eradication of Acinetobacter baumannii/Enterobacter cloacae complex in an open proximal tibial fracture and closed drop foot correction with a multidisciplinary approach using the Taylor Spatial Frame®: a case report, Eur. J. Med. Res., 24, 2, https://doi.org/10.1186/s40001-019-0360-2, 2019.
Steinmetz, S., Wernly, D., Moerenhout, K., Trampuz, A., and Borens, O.: Infection after fracture fixation, EFORT Open Rev., 4, 468–475, https://doi.org/10.1302/2058-5241.4.180093, 2019.
Titécat, M. Senneville, E., Wallet, E., Dezèquec, H., Migaud, H., Courcol, R.-J., and Loïez, C.: Bacterial epidemiology of osteoarticular infections in a referent center: 10-year study, Orthopedics and Traumatology: Surgery and Research, 99, 653–658, https://doi.org/10.1016/j.otsr.2013.02.011, 2013.
Trampuz, A. and Zimmerli, W.: Diagnosis and treatment of infections associated with fracture-fixation devices, Injury, 37, S59–66, https://doi.org/10.1016/j.injury.2006.04.010, 2006.
Tschudin-Sutter, S., Frei, R., Dangel, M. Jacob, M., Balmelli, C., and Schaefer, D. J.: Validation of a treatment algorithm for orthopaedic implant-related infections with device-retention-results from a prospective observational cohort study, Clin. Microbiol. Infect., 22, 457.e1–457.e9, https://doi.org/10.1016/j.cmi.2016.01.004, 2016.
Uçkay, I., Jugun, K. Gamulin, A., Wagener, J., Hoffmeyer, P., and Lew, D.: Chronic osteomyelitis, Curr. Infect. Dis. Rep., 14, 566–575, https://doi.org/10.1007/s11908-012-0286-0, 2012.
Valour, F., Dutronc, H., Dinh, A., Cazorla, C., Pavèse, P., Lesens, O., Uçkay, I., Chidiac, C., Ferry, T., and Colistin BJIs Study Group: Difficult to treat Gram-negative bone and joint infections: efficacy and safety of prolonged intravenous colistin, Int. J. Antimicrob. Agents, 41, 197–207, https://doi.org/10.1016/j.ijantimicag.2012.09.016, 2013.