the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Implant surface modifications as a prevention method for periprosthetic joint infection caused by Staphylococcus aureus: a systematic review and meta-analysis
Krisna Yuarno Phatama
Respati Suryanto Dradjat
Edi Mustamsir
Dwi Yuni Nurhidayati
Dewi Santosaningsih
Dwikora Novembri Utomo
Mohamad Hidayat
Background: Periprosthetic joint infection is the most common infection due to joint replacement. It has been reported that, over a 5-year time span, 3.7 % of cases occurred annually. This statistic has increased to 6.86 % over 16 years. Thus, an effective method is required to reduce these complications. Several strategies such as coating methods with various materials, such as antibiotics, silver, and iodine, have been reported. However, the best preventive strategy is still undetermined. Therefore, this systematic review aims to evaluate the outcome of coating methods on joint arthroplasty as a treatment or preventive management for infection complications. Methods: Eligible articles were systematically searched from multiple electronic databases (PubMed, Cochrane library, and ScienceDirect) up to 2 June 2022. Based on the criterion inclusion, eight articles were selected for this study. The Newcastle–Ottawa scale (NOS) was used to assess the quality of the study, and the meta-analysis test was conducted with Review Manager 5.4. Results: The quality of the articles in this study is in the range of moderate to good. It was found that the application of modified antibiotic coatings significantly reduced the occurrence of periprosthetic joint infection (PJI) (p 0.03), and silver coating could not significantly (p 0.47) prevent the occurrence of PJI. However, according to the whole aspect of coating modification, the use of antibiotics, silver, and iodine can minimize the occurrence of PJI (p <0.0001). Conclusion: Coating methods using antibiotics are an effective method that could significantly prevent the occurrence of PJI. On the other hand, coating with non-antibiotic materials such as silver could not significantly prevent the incidence of PJI.
- Article
(1107 KB) - Full-text XML
- BibTeX
- EndNote
Periprosthetic joint infection (PJI) is defined as a complication obtained after joint arthroplasty and is characterized by implant tissue infection of the artificial joint. The incidence of PJIs ranged from 11 % to 14 % for hip procedure revisions and from 11 % to 25 % for knee revisions (Reina et al., 2013; Kapadia et al., 2016). In addition, the increasing number of elderly populations has resulted in a higher demand for patients to be functional even in old age. This condition has also influenced the steadily rising number of joint replacement surgeries (Wengler et al., 2014). However, the complications after the artificial joint replacement represent significant challenges for the patient and the attending physician, especially for knee arthroplasties (Wetters et al., 2013).
The most common cause of PJI is Staphylococcus aureus. More than half of all PJI cases are caused by this bacterium (Cobo and Del Pozo, 2011). PJI can also be caused by the Staphylococcus epidermidis group, Enterococcus sp., and Gram-negative bacilli, but in small incidences (Nair et al., 2017). These bacteria induce PJI by forming a biofilm that is a form of adaptive bacterial response to various stressors that provide crucial protection to bacteria (Lamret et al., 2020).
Nowadays, important strategies to prevent and reduce complications due to PJI are widely researched. PJI prevention strategies consist of dental prophylaxis, antibiotic prophylaxis, and surface modification with an active or passive coating such as silver coat, hydrogen coat, chlorine, iodine coat, or chromium coat (Beyer et al., 2016; Arciola et al., 2018; Sebastian et al., 2020; Fiore et al., 2021). However, when PJI has occurred, only a few approaches have been reported as successfully managing the PJI and reduced complications, including debridement, antibiotics, and implant retention (DAIR) (Vaz et al., 2020) and first- or second-stage surgical revision (Kunutsor et al., 2015). Although these prevention and treatment strategies have been conducted properly, the incidence of PJI remains to be increased (Zmistowski and Casper, 2013; Gundtoft et al., 2017).
For the PJI pathway, inhibiting biofilm formation by bacteria has proven to be an important prevention strategy (Tzeng et al., 2015). Therefore, the implant's surface has been proposed as a location for modifications for the development of antibacterial approaches, because it is where the biofilm arises (Bumgardner et al., 2011). Thus, it is necessary to conduct an in-depth study of the effectiveness of prevention, one of which is by using the coating method to obtain the most optimal outcome. In addition, the outcomes of prospective/retrospective prevention research are still inconsistent. The use of coatings in primary arthroplasty in particular is still a point of disagreement. Therefore, this systematic review evaluates the outcome of coating methods on arthroplasty as a treatment or form of preventive management.
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline.
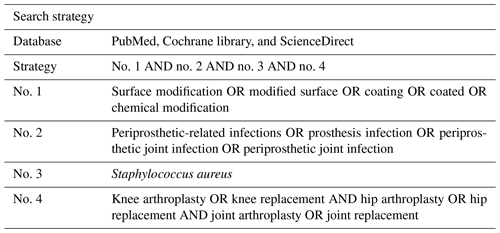
2.1 Data sources and search strategy
Eligible articles were systematically searched from multiple electronic databases (PubMed, Cochrane library, and ScienceDirect) up to 2 June 2022. The search strategy applied used the Boolean methods outlined in Table 1. The selected articles and their relevant reference materials were assessed according to the eligibility criteria. Initially, the articles selected were available in full text from an electronic search, published within 10 years, and only in English.
2.2 Selection criteria
The types of articles included were randomized clinical trials (RCTs), cohort studies, as well as cross-sectional and case-control research that reported PJI after knee or hip arthroplasty. Review articles, comment editorials, animal model studies, and cadaveric investigations were all excluded from the study. The type of article and evidence level is considered a source in this study. Therefore, case reports/series were excluded due to the low level of evidence. Next, unpublished papers and articles relevant to the incidence of PJI but that did not use coating methods were excluded. Remnant articles that included studies and results regarding subjects of relevance were collected and summarized. Then, the data of search results were tabulated into Excel, and Mendeley was used to eliminate the duplicate studies.
2.2.1 Participants
The studies selected contained patients that underwent joint replacement surgery, either TKA (total knee arthroplasty) or THA (total hip arthroplasty).
2.2.2 Intervention
In this study, the interventions used included patients who underwent joint replacement and were given a coating on the implant. There was no limitation on the coating materials that were used.
2.2.3 Outcome
The incidence of PJI is the primary outcome, and the duration of follow-up was not a consideration in this study. The definition and diagnostic criteria of PJI followed MSIS guidelines and the 2018 definition of PJI (Osmon et al., 2013; Parvizi et al., 2018).
2.3 Quality assessment
Two reviewers (KY and EM) objectively reviewed the potential articles included in this study. The Newcastle–Ottawa scale (NOS) was used to evaluate the case control, cohort, and RCT study quality. For any disagreements, the senior author (RS) made the final opinion. However, any discrepancies were solved by a consensus view. When the NOS was used to assess the study's quality, the article was given a score, with the lowest being 0 and the highest being 9. The study's quality was classified as excellent (score of 7), good (score of 5–6), and poor (score of 4).
2.4 Data synthesis and analysis
Continuous variables were presented as even or incidences of PJI for the comparison between coating and uncoating. Statistical significance was defined as a P value of 0.05. Windows Review Manager (RevMan) 5.4.1 was used to conduct the meta-analysis. If at least two studies focused on the same factors, a meta-analysis evaluated whether a random or fixed effect based on heterogeneity was found.
3.1 Articles' inclusion characteristics
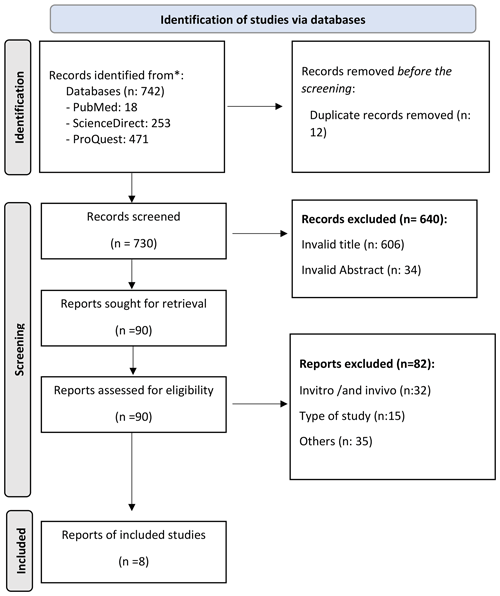
The search process through eligible research articles from multiple electronic databases found 742 articles, of which 18 were from PubMed, 253 were from Science, and 471 were from ProQuest. After sorting the duplicate articles, we excluded 12, and 730 remained. The following process consisted of evaluating the titles and abstract based on the set inclusion criteria, which resulted in 90 remaining articles. After reviewing the content of the articles, 82 articles were excluded. As a result, eight articles were used in this systematic review (Fig. 1).
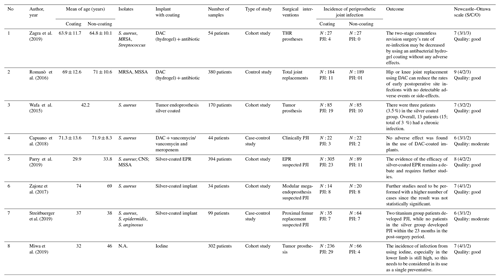
Among the eight articles, six were cohort studies, and two were case-control studies. The total number of patients included in this study is 1477 patients. Various coating materials were used to prevent the incidence of PJI. In this study, four studies used silver coating, three studies used antibiotics, and one used iodine. All articles contained Staphylococcus aureus samples from PJI patients (Table 5).
According to the Newcastle–Ottawa scale, all of the selected studies' experimental methods matched the research criteria, of which five were of good quality and three were of moderate quality. Consequently, this study was considered reasonable, with a minimal risk of bias (Table 5).
3.2 Clinical studies
The coatings used to prevent PJI vary hugely, with several studies using DAC (defensive antibacterial coating) combined antibiotics and even iodine. However, it is reported that these coatings are ineffective, as there are cases where patients would suffer from PJI and prosthesis failure. The incidence of PJI in the experimental group that used an DAC combined antibiotic coating (hydrogel) on the prosthesis surface has been shown to be an effective method of preventing PJI compared to the control group. The incidence of PJI in the antibiotic coating group was reported to have happened in 3 patients, while in the control group, PJI occurred in 18 patients (Romanò et al., 2016; Capuano et al., 2018; Zagra et al., 2019).
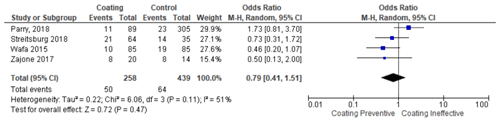
In contrast, when silver was used as the base-coating material, there was still a high rate of PJI. Of the total of 258 patients who were given a silver coating, 36 patients (13.9 %) had PJI, while for patients without the silver coating, 57 reported having PJI (Wafa et al., 2015; Zajonz et al., 2017; Parry et al., 2019; Streitbuerger et al., 2019).
3.3 Antibiotic coating
In this study, DAC was mostly combined with antibiotics such as gentamicin sulfate combined with vancomycin, meropenem, vancomycin only, teicoplanin, ceftazidime, or vancomycin + rifampicin/vancomycin meropenem. Combinations depend on surgeon preference and bacterial maps based on antibiograms from operator institutions as well as multidisciplinary support from institutional infection control specialists. Zagra et al. (2019) reported that, in their research, there was no adverse effect in the DAC group combined with antibiotics. In contrast, in the control group, infection was found in four patients. In line with the previous study, Romanò et al. (2016) performed follow-ups for 24 months. They concluded that DAC, which is combined with antibiotics, could reduce the rates of early postoperative site infections, with no detectable adverse events or side-effects during the follow-up. The clinical studies showed how combining PJI with antibiotics effectively prevented and treated PJI. Moreover, one of the important factors in preventing PJI re-infection was considering the combination of antibiotics. Despite the promising outcome of administering antibiotics as a preventive treatment, there are still several problems, such as resistance, re-infection, and implant failure.
In other studies, Capuano et al. (2018) compared the outcome of a one-stage revision procedure combined with antibiotics against a two-stage procedure without coating in patients with PJI. Their study reported that two patients in the one-stage procedure and three in the two-stage procedure had a recurrence of infection. In the study's conclusion, they argued that two-stage revision without coating and one-stage exchange with DAC-coated implants showed a similar incidence of infection recurrence.
3.4 Silver coating
A study by Parry et al. (2019) compared silver coating and non-silver coating prostheses and found that there was no significant difference between the two groups. This is in line with a previous study conducted by Zajonz et al. (2017), who reported that there was no statistically significant difference between silver coating against non-silver coating prosthesis. However, both studies have the same conclusion that silver's potential as an antimicrobial is still debatable. Conversely, Streitbuerger et al. (2019) found a different result. The study reported that infection happened in 14 % and 9.4 % of titanium and silver groups, respectively. Moreover, the infection rate in the silver group was 10.9 %.
3.5 Iodine coating
Miwa et al. (2019) evaluated the outcome of the incidence of SSI (surgical site infection) using iodine coating. They reported that the incidence of SSI on 33 of 302 patients with an infection in the femur and tibia was 25.0 % and 2.8 %, respectively. They reported that 4 patients (6.1 %) out of 66 had SSI after coating with antibiotics, whereas in the uncoated group, 29 (12.2 %) out of 236 had SSI.
3.6 Statistic test results
The meta-analysis was conducted in two stages. In the first stage, the coating group that uses various materials (hydrogel, silver, and iodine) was compared with the non-coating group (control group). From these steps, it was found that the use of coatings, in general, prevented the occurrence of PJI and was statistically significant (p <0.0001) (citation locations from Table 2). Moreover, it was found that the infection rate was lowered in seven studies compared to their respective control group. Then, in the second stage, the studies were divided into different subgroups. The test result of the antibiotic coating group found that the antibiotic coating potentially reduced the incidence of periprosthetic infection, with significant test results (p 0.03) with a homogeneous data distribution (Table 3). Next, the silver coating group test result found that the silver coating also potentially prevents the incidence of PJI. Unfortunately, the meta-analysis test result was not statistically significant (p 0.47). Therefore, silver cannot be fully used broadly as a single modality (Table 4).
Table 2Forest plot showing the use of coating vs. uncoating methods on the implant surface in the occurrence of PJI.
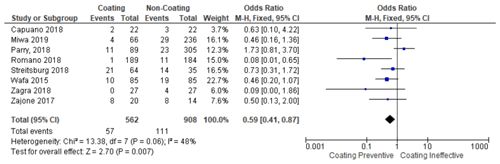
Table 3Forest plot sub-group for the use of coating methods using antibiotics on the implant surface on the occurrence of PJI.
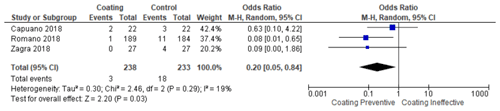
Table 4Forest plot sub-group for the use of coating methods using silver on the implant surface on the occurrence of PJI.
Joint replacement with prosthetics is one of the most important breakthroughs in the development of orthopedic surgery today. In the medium and long term, joint replacement can significantly reduce pain, improve quality of life, and increase the mobility of the patient (Meftah et al., 2016). However, when endoprosthesis or artificial joints (hips, knees, elbows, and ankles) have been placed in the body, they can potentially cause a hypersensitivity reaction, an inflammatory response, which can cause infection when there is a lack of aseptic prevention (Beam and Osmon, 2018). The periprosthetic infection problem remains a significant and devastating consequence. However, over the years, several efforts have been made. The American Academy of Orthopedic Surgeons (AAOS) has created recommendations for minimizing the incidence of PJI by reducing the risk factors of PJI such as weight loss, treating toothaches, controlling diabetes, conducting a blood laboratory examination before TKA or THA (ESR, CRP, IL-6), giving antibiotic prophylaxis before surgery (cephalosporin, glycopeptide), and conducting preventive durante operation such as washing the wound area with an antiseptic and povidone–iodine solution (Tubb et al., 2020). Unfortunately, despite the efforts taken, the infection rate has remained relatively constant, and PJI continues to happen (Li et al., 2018; Sambri et al., 2019).
The causative pathogens for PJI are mostly coagulase-negative staphylococci, Staphylococcus aureus, streptococci, enterococci, and Gram-negative bacteria (Lamagni, 2014). The pathogens would have either entered the joint during the operation or later as part of bacteremia or spread from a neighboring site of infection (Osmon et al., 2013). Therefore, before undergoing surgical treatment, systemic antibiotic therapy is also mandatory. No matter the type of surgery, antibiotics should be administered for 2–6 weeks (Li et al., 2018). Previous studies have reported that the use of systemic antibiotics as prophylaxis can significantly reduce the infection rate (Romanò et al., 2015). Other clinical studies have shown that, when combined with systemic antibiotic therapy, antibiotic-loaded bone cement can reduce the risks of deep infection in cemented total hip arthroplasty procedures and revisions because of alleged “aseptic” loosening (Romanò et al., 2016).
Understanding the diagnosis and conducting early treatment are essential for preventing the incidence of PJI. However, this initiative is deemed insufficient, and it is suggested that coating the prosthesis is an effective preventative strategy. One of the promising techniques to reduce infection is prophylaxis as an implant surface modification. It uses various base materials such as DAC combined with antibiotics, silver (Zajonz et al., 2017; Miwa et al., 2019; Streitbuerger et al., 2019), chromium (Beyer et al., 2016), and iodine (Miwa et al., 2019). The coating aims to break through the biofilm to preserve the prosthesis. A latency period of 2–4 weeks after the onset of infection is given for this type of infection (Osmon et al., 2013; Minassian et al., 2014). According to experts' opinion, biofilm formation can be regarded as complete for infections that are treated later. Nowadays, various strategies are used to prevent biofilm formation. One is by inhibiting the quorum sensing mechanism by using coating methods on prosthetic surfaces (Tzeng et al., 2015).
The antibiotic coating strategy helps to prevent deep infection in spine procedures and is effective in joint arthroplasty (Xiong et al., 2014; Gallo et al., 2016). This statement is in line with the result of studies that reported that an antibiotic coating is predominant in preventing the occurrence of PJI. Nevertheless, joint replacement infections persist in approximately 1 %–2 % of cases.
The antibiotic coating used in this study mostly was adopted from DAC® hydrogel instruction (Romanò et al., 2016; Zagra et al., 2019; Capuano et al., 2018). The DAC, composed of hyaluronic acid and polylactic acid, was administered with a prefilled syringe containing 300 mg of sterile DAC powder mixed with a solution of 5 mL sterile water for injection. The process to obtain the desired antibiotic-loaded hydrogel took approximately 3 to 5 min, the antibiotic-loaded hydrogel at a DAC concentration of 6 % (w/v – weight/volume percentage concentration), and an antibiotic concentration ranging from 20 to 50 mg mL−1, depending on the surgeon's choice. A few minutes after reconstitution, the hydrogel was applied directly to the implant, which was subsequently put into the body according to standard procedure (Romanò et al., 2015). In other cases, surgeons can choose another antibiotic to reflect the antibiogram of the individual institution, the individual risk factors of the patient, and multidisciplinary support of institutional infection control experts.
The results of the meta-analysis test showed that the coating method using DAC combined with antibiotics caused the occurrence of PJI in 3 patients out of a total of 471 patients. Therefore, this result is statistically significant (p 0.03). For this reason, this result proves that antibiotics are highly recommended for use as an effort to prevent PJI. However, the effectiveness of antibiotics can cause a big problem of side-effects as well (Kapadia et al., 2016). When using antibiotics as modified implant material in a clinical situation, there are at least two things to consider. The first aspect is the variety of pathogens that are linked with PJI. Staphylococcus aureus and Staphylococcus epidermidis are the two major pathogens that cause PJI (Aggarwal et al., 2014; Holleyman et al., 2016). The second factor is antibiotic resistance. Anagnostakos and Sahan (2021) performed a study to test the resistance level of patients treated with a coating. This study showed that 54.2 % of S. aureus and S. epidermidis were resistant to clindamycin, whereas 37.1 % were resistant to gentamicin and showed susceptibility against vancomycin (Anagnostakos and Sahan, 2021).
Then, the results of the meta-analysis study which compared the coatings with non-coatings generally showed a significant value (p 0.007). On the other hand, the heterogeneity of these data is high (I2: 48 %). This is most likely due to variations in age and gender across all studies. Moreover, there are differences in the length of follow-up in each study, and this can also affect the outcome of this research.
Another coating method that has been reported is the use of silver. In this study, we found that silver used as part of a prevention strategy against PJIs is not yet ideal. Of the 258 silver-coated patients, 50 (19.3 %) developed an infection in the implants. In addition, the results of the meta-analysis test did not show significant results with p of 0.47. Therefore, the use of silver coating as a prevention method for PJI still needs to be considered.
Furthermore, some studies stated that there is no difference between silver coating for patients with high-risk PJI and non-silver coating for patients with primary bone tumor prostheses (Zajonz et al., 2017; Parry et al., 2019). In another study, Streitbuerger et al. (2019) reported that silver coating does not generally inhibit the incidence of PJI. However, when an infection occurs, the silver coating has the potential to reduce the possibility of a two-stage prosthesis change (Xie et al., 2011; Mahamuni-Badiger et al., 2020). Additionally, only one study used iodine as the base material for the coating method. Therefore, the meta-analysis test cannot be conducted. The use of iodine showed that it is as effective as silver in preventing the incidence of PJI. In this study, when iodine was used as a coating, 2 patients out of a total of 44 patients, or about 4.5 %, experienced PJI (Miwa et al., 2019).
This study has provided a comprehensive explanation regarding the outcomes of using coatings to prevent PJI. However, there are limitations to this study, such as the majority of the type of research used being a cohort/cross-sectional study. Therefore, the level of evidence is classified as moderate. For future research suggestions, RCT studies need to be explored to obtain a better level of evidence. It is also necessary to explore non-antibiotic coating materials other than silver and iodine to find other effective coatings that could be used as an alternative to antibiotics.
Antibiotic coatings are an effective method that could significantly prevent the occurrence of PJI. However, some side-effects were reported, such as antibiotic resistance, which led to the recurrence of infection. The use of non-antibiotics such as silver and iodine as coating materials resulted in a relatively high incidence of PJI and could not significantly prevent the occurrence of PJI.
The data that support the findings of this study are not openly available and are available from the corresponding author upon reasonable request.
The additional information used in this work can be accessed by contacting Krisna Yuarno Phatama (Krisnayuarno@ub.ac.id).
KYP developed the theory, drafted the manuscript, and collected data. DYN and DS performed the computations, collected data, interpreted the data and verified the analytical methods and results. MH, RSD, EM, and DNU developed the theory, supervised the findings of this work, and conceived the study. All the authors discussed the results and contributed to the final manuscript.
The contact author has declared that none of the authors has any competing interests.
An ethics statement is not applicable because this study is based exclusively on published literature.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research paper is made possible by God’s grace and through the support from parents, family, and friends.
This paper was edited by Bryan Springer and reviewed by two anonymous referees.
Aggarwal, V. K., Bakhshi, H., Ecker, N. U., Parvizi, J., Gehrke, T., and Kendoff, D.: Organism profile in periprosthetic joint infection: pathogens differ at two arthroplasty infection referral centers in Europe and in the United States, J. Knee Surg., 27, 399–406, https://doi.org/10.1055/s-0033-1364102, 2014.
Anagnostakos, K. and Sahan, I.: Are cement spacers and beads loaded with the correct antibiotic(s) at the site of periprosthetic hip and knee joint infections?, Antibiotics, 10, 143, https://doi.org/10.3390/antibiotics10020143, 2021.
Arciola, C. R., Campoccia, D., and Montanaro, L.: Implant infections: Adhesion, biofilm formation and immune evasion, Nat. Rev. Microbiol., 16, 397–409, https://doi.org/10.1038/s41579-018-0019-y, 2018.
Beam, E. and Osmon, D.: Prosthetic Joint Infection Update, Infect. Dis. Clin. North Am., 32, 843–859, https://doi.org/10.1016/j.idc.2018.06.005, 2018.
Beyer, F., Lützner, C., Kirschner, S., and Lützner, J.: Midterm results after coated and uncoated TKA: A randomized controlled study, Orthopedics, 39, S13–S17, https://doi.org/10.3928/01477447-20160509-10, 2016.
Bumgardner, J. D., Adatrow, P., Haggard, W. O., and Norowski, P. A.: Emerging antibacterial biomaterial strategies for the prevention of peri-implant inflammatory diseases, Int. J. Oral Maxillofac. Implants, 26, 553–560, 2011.
Capuano, N., Logoluso, N., Gallazzi, E., Drago, L., and Romanò, C. L.: One-stage exchange with antibacterial hydrogel coated implants provides similar results to two-stage revision, without the coating, for the treatment of peri-prosthetic infection, Knee Surgery, Sport. Traumatol. Arthrosc., 26, 3362–3367, https://doi.org/10.1007/s00167-018-4896-4, 2018.
Cobo, J. and Del Pozo, J. L.: Prosthetic joint infection: diagnosis and management., Expert Rev. Anti. Infect. Ther., 9, 787–802, https://doi.org/10.1586/eri.11.95, 2011.
Fiore, M., Sambri, A., Zucchini, R., Giannini, C., Donati, D. M., and De Paolis, M.: Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: a systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery, Eur. J. Orthop. Surg. Traumatol., 31, 201–220, https://doi.org/10.1007/s00590-020-02779-z, 2021.
Gallo, J., Panacek, A., Prucek, R., Kriegova, E., Hradilova, S., Hobza, M., and Holinka, M.: Silver Nanocoating Technology in the Prevention of Prosthetic Joint Infection, Mater. (Basel, Switzerland), 9, 337, https://doi.org/10.3390/ma9050337, 2016.
Gundtoft, P. H., Pedersen, A. B., Varnum, C., and Overgaard, S.: Increased Mortality After Prosthetic Joint Infection in Primary THA, Clin. Orthop. Relat. Res., 475, 2623–2631, https://doi.org/10.1007/s11999-017-5289-6, 2017.
Holleyman, R. J., Baker, P., Charlett, A., Gould, K., and Deehan, D. J.: Microorganisms responsible for periprosthetic knee infections in England and Wales, Knee Surg. Sports Traumatol. Arthrosc., 24, 3080–3087, https://doi.org/10.1007/s00167-015-3539-2, 2016.
Kapadia, B. H., Berg, R. A., Daley, J. A., Fritz, J., Bhave, A., and Mont, M. A.: Periprosthetic joint infection, Lancet, 387, 386–394, https://doi.org/10.1016/S0140-6736(14)61798-0, 2016.
Kunutsor, S. K., Whitehouse, M. R., Blom, A. W., Beswick, A. D., Strange, S., Garfield, K., Lenguerrand, E., Gooberman-Hill, R., Moore, D., Burston, A., Simon, J., King, G., Wylde, V., Noble, S., Lane, A., Carroll, F., Webb, J., MacGowan, A., Jones, S., Taylor, A., Dieppe, P., Toms, A., Wilson, M., Stockley, I., Burston, B., Whittaker, J. P., and Board, T.: Re-infection outcomes following one- and two-stage surgical revision of infected hip prosthesis: A systematic review and meta-analysis, PLoS One, 10, 1–14, https://doi.org/10.1371/journal.pone.0139166, 2015.
Lamagni, T.: Epidemiology and burden of prosthetic joint infections, J. Antimicrob. Chemother., 69, 5–10, https://doi.org/10.1093/jac/dku247, 2014.
Lamret, F., Colin, M., Mongaret, C., Gangloff, S. C., and Reffuveille, F.: Antibiotic tolerance of Staphylococcus aureus biofilm in periprosthetic joint infections and antibiofilm strategies, Antibiotics, 9, 547, https://doi.org/10.3390/antibiotics9090547, 2020.
Li, C., Renz, N., and Trampuz, A.: Management of Periprosthetic Joint Infection, Hip Pelvis, 30, 138–146, https://doi.org/10.5371/hp.2018.30.3.138, 2018.
Mahamuni-Badiger, P. P., Patil, P. M., Badiger, M. V., Patel, P. R., Thorat-Gadgil, B. S., Pandit, A., and Bohara, R. A.: Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles, Mat. Sci. Eng. C-Biomim., 108, 110319, https://doi.org/10.1016/j.msec.2019.110319, 2020.
Meftah, S., Belhaj, K., Zahi, S., Mahir, L., lmidmani, F., and El Fatimi, A.: Comparison of functional outcomes and quality of life after THA according to indication about 210 THA, Ann. Phys. Rehabil. Med., 59, e111, https://doi.org/10.1016/j.rehab.2016.07.247, 2016.
Minassian, A. M., Osmon, D. R., and Berendt, A. R.: Clinical guidelines in the management of prosthetic joint infection, J. Antimicrob. Chemother., 69, 29–35, https://doi.org/10.1093/jac/dku253, 2014.
Miwa, S., Shirai, T., Yamamoto, N., Hayashi, K., Takeuchi, A., Tada, K., Kajino, Y., Higuchi, T., Abe, K., Aiba, H., Taniguchi, Y., and Tsuchiya, H.: Risk factors for surgical site infection after malignant bone tumor resection and reconstruction 11 Medical and Health Sciences 1103 Clinical Sciences, BMC Cancer, 19, 33, https://doi.org/10.1186/s12885-019-5270-8, 2019.
Nair, R., Schweizer, M. L., and Singh, N.: Septic Arthritis and Prosthetic Joint Infections in Older Adults, Infect. Dis. Clin. North Am., 31, 715–729, https://doi.org/10.1016/j.idc.2017.07.013, 2017.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., and Wilson, W. R.: Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America., Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am., 56, e1–e25, https://doi.org/10.1093/cid/cis803, 2013.
Parry, M. C., Laitinen, M. K., Albergo, J. I., Gaston, C. L., Stevenson, J. D., Grimer, R. J., and Jeys, L. M.: Silver-coated (Agluna®) tumour prostheses can be a protective factor against infection in high risk failure patients, Eur. J. Surg. Oncol., 45, 704–710, https://doi.org/10.1016/j.ejso.2018.12.009, 2019.
Parvizi, J., Tan, T. L., Goswami, K., Higuera, C., Della Valle, C., Chen, A. F., and Shohat, N.: The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria, J. Arthroplasty, 33, 1309–1314.E2, https://doi.org/10.1016/j.arth.2018.02.078, 2018.
Reina, N., Delaunay, C., Chiron, P., Ramdane, N., and Hamadouche, M.: Infection as a cause of primary total hip arthroplasty revision and its predictive factors, Orthop. Traumatol. Surg. Res., 99, 555–561, https://doi.org/10.1016/j.otsr.2013.07.001, 2013.
Romanò, C. L., Scarponi, S., Gallazzi, E., Romanò, D., and Drago, L.: Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama, J. Orthop. Surg. Res., 10, 157, https://doi.org/10.1186/s13018-015-0294-5, 2015.
Romanò, C. L., Malizos, K., Capuano, N., Mezzoprete, R., D'Arienzo, M., Der, C. V., Scarponi, S., and Drago, L.: Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty?, J. Bone Joint Infect., 1, 34–41, https://doi.org/10.7150/jbji.15986, 2016.
Sambri, A., Bianchi, G., Parry, M., Frenos, F., Campanacci, D., Donati, D., and Jeys, L.: Is Arthrodesis a Reliable Salvage Option following Two-Stage Revision for Suspected Infection in Proximal Tibial Replacements? A Multi-Institutional Study, J. Knee Surg., 32, 911–918, https://doi.org/10.1055/s-0038-1672121, 2019.
Sebastian, S., Liu, Y., Christensen, R., Raina, D. B., Tägil, M., and Lidgren, L.: Antibiotic containing bone cement in prevention of hip and knee prosthetic joint infections: A systematic review and meta-analysis, J. Orthop. Transl., 23, 53–60, https://doi.org/10.1016/j.jot.2020.04.005, 2020.
Streitbuerger, A., Henrichs, M. P., Hauschild, G., Nottrott, M., Guder, W., and Hardes, J.: Silver-coated megaprostheses in the proximal femur in patients with sarcoma, Eur. J. Orthop. Surg. Traumatol., 29, 79–85, https://doi.org/10.1007/s00590-018-2270-3, 2019.
Tubb, C. C., Polkowksi, G. G., and Krause, B.: Diagnosis and Prevention of Periprosthetic Joint Infections, J. Am. Acad. Orthop. Surg., 28, E340–E348, https://doi.org/10.5435/JAAOS-D-19-00405, 2020.
Tzeng, A., Tzeng, T. H., Vasdev, S., Korth, K., Healey, T., Parvizi, J., and Saleh, K. J.: Treating periprosthetic joint infections as biofilms: Key diagnosis and management strategies, Diagn. Microbiol. Infect. Dis., 81, 192–200, https://doi.org/10.1016/j.diagmicrobio.2014.08.018, 2015.
Vaz, K., Scarborough, M., Bottomley, N., Kendrick, B., Taylor, A., Price, A., Alvand, A., and Jackson, W.: Debridement, antibiotics and implant retention (DAIR) for the management of knee prosthetic joint infection, Knee, 27, 2013–2015, https://doi.org/10.1016/j.knee.2020.08.011, 2020.
Wafa, H., Grimer, R. J., Reddy, K., Jeys, L., Abudu, A., Carter, S. R., and Tillman, R. M.: Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: Case-control study, Bone Jt. J., 97-B, 252–257, https://doi.org/10.1302/0301-620X.97B2.34554, 2015.
Wengler, A., Nimptsch, U., and Mansky, T.: Hüft- und kniegelenkersatz in Deutschland und den USA: Auswertung deutscher und us-amerikanischer krankenhauseinzelfalldaten von 2005 bis 2011, Dtsch. Arztebl. Int., 111, 407–416, https://doi.org/10.3238/arztebl.2014.0407, 2014.
Wetters, N. G., Murray, T. G., Moric, M., Sporer, S. M., Paprosky, W. G., and Della Valle, C. J.: Risk factors for dislocation after revision total hip arthroplasty hip, Clin. Orthop. Relat. Res., 471, 410–416, https://doi.org/10.1007/s11999-012-2561-7, 2013.
Xie, Y., He, Y., Irwin, P. L., Jin, T., and Shi, X.: Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni, Appl. Environ. Microbiol., 77, 2325–2331, https://doi.org/10.1128/AEM.02149-10, 2011.
Xiong, L., Pan, Q., Jin, G., Xu, Y., and Hirche, C.: Topical intrawound application of vancomycin powder in addition to intravenous administration of antibiotics: A meta-analysis on the deep infection after spinal surgeries, Orthop. Traumatol. Surg. Res., 100, 785–789, https://doi.org/10.1016/j.otsr.2014.05.022, 2014.
Zagra, L., Gallazzi, E., Romanò, D., Scarponi, S., and Romanò, C.: Two-stage cementless hip revision for peri-prosthetic infection with an antibacterial hydrogel coating: results of a comparative series, Int. Orthop., 43, 111–115, https://doi.org/10.1007/s00264-018-4206-2, 2019.
Zajonz, D., Birke, U., Ghanem, M., Prietzel, T., Josten, C., Roth, A., and Fakler, J. K. M.: Silver-coated modular Megaendoprostheses in salvage revision arthroplasty after periimplant infection with extensive bone loss – A pilot study of 34 patients, BMC Musculoskelet. Disord., 18, 383, https://doi.org/10.1186/s12891-017-1742-7, 2017.
Zmistowski, B. and Casper, D. S.: Periprosthetic Joint Infection Increases the Risk, J. Bone Joint Surg. Am., 95, 2177–2185, https://doi.org/10.2106/JBJS.L.00789, 2013.