the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Utility of disc space aspirate cell counts and differentials in the diagnosis of native vertebral osteomyelitis
Talha Riaz
Matthew Howard
Felix Diehn
Aaron Joseph Tande
Courtney Ross
Paul Huddleston
Elie Berbari
Background: Aspiration of intervertebral disc space is often done to confirm the diagnosis of native vertebral osteomyelitis. A study has not been done examining the utility of cell counts and differentials of the aspirated fluid in diagnosing native vertebral osteomyelitis (NVO). Methods: In this feasibility study, we prospectively enrolled patients with a suspected diagnosis of NVO referred to the Division of Neuroradiology for image-guided needle aspiration of the intervertebral disc. In this study, manual cell count was done on the aspirated fluid, followed by a differential cytospin technique and touch prep. We obtained demographic, lab, and microbiologic data and used the receiver operating curve (ROC) for statistical analysis. Results: Over 12 months, we performed 17 aspirates on 14 patients. The median age was 70.5 years (range: 45–77). The median manual cell count on the aspirated fluid was 52 cells µL−1 (range: 0–6656), the median neutrophil percentage on the touch prep slide was 73 % (range: 5 %–100 %), and the median neutrophil percentage on the cytospin slide was 82 % (range: 0 %–100 %). Routine bacterial cultures were positive in five cases, and the 16S ribosomal RNA gene polymerase chain reaction was positive in two cases. The optimal cutoff for a cell count of 104 total nucleated cells offered a sensitivity and specificity of 86 %, and a neutrophil cutoff of 83 % was associated with a 71 % sensitivity and specificity. Conclusion: An image-guided aspirated specimen leukocyte differential of ≥83 % neutrophils or a leukocyte count of ≥104 µL−1 was a sensitive and specific test for diagnosing patients with suspected NVO. Additionally, more extensive studies are warranted to confirm the findings.
- Article
(571 KB) - Full-text XML
- BibTeX
- EndNote
Between 1998 and 2013, native vertebral osteomyelitis (NVO) admission in the United States increased from to (Issa et al., 2018). A population-based review of all patients with osteomyelitis in Olmsted County, Minnesota, in the Rochester Epidemiology Project, revealed a similar disease burden, with the age- and sex-adjusted incidence of vertebral osteomyelitis increasing from 0.5 cases per 100 000 person-years in the period from 1969 to 1979 to 4.7 cases per 100 000 person-years in the period from 2000 to 2009 (Kremers et al., 2015). NVO tends to have an indolent course (Eren Gok et al., 2014), and diagnosis is often delayed (Nickerson and Sinha, 2016; Zimmerli, 2010). NVO is associated with substantial morbidity and mortality, and delay in the diagnosis can lead to complications, including the formation of paravertebral or epidural abscess, paralysis, or death (Mylona et al., 2009; Zimmerli, 2010).
Infectious Diseases Society of America (IDSA) guidelines recommend obtaining an MRI of the spine and blood cultures in patients with symptoms suggestive of NVO (Berbari et al., 2015). An image-guided disc sample is recommended if blood cultures remain without growth after 48 h (Babic and Simpfendorfer, 2017; Shaltot et al., 1982). The positivity rate of microbiology from an image-guided biopsy remains variable, 14 % to 76 % in pyogenic vertebral osteomyelitis (Nickerson and Sinha, 2016) and 42 %–76 % in tuberculous VO (Colmenero et al., 2013). In a recent retrospective study comprising 77 patients with NVO, sample culture was positive in 43 (56 %) (Fragio Gil et al., 2022). However, in another recent study by Wong et al. (2021), patients without prior antibiotic use had a similar microbiological yield compared to those on antibiotics at the time of CT-guided biopsy for NVO (Wong et al., 2021).
The low sensitivity of the microbiologic culture obtained via image-guided needle sampling reported in retrospective cohort studies is partially due to prior administration of antimicrobial therapy or sampling errors (Rankine et al., 2004). A repeat image-guided sample is recommended in patients with an initial culture-negative biopsy, but the additional yield of a second sample is only between 14 % and 60 % (Czuczman et al., 2018). Methods to enhance the accuracy of image-guided sampling are warranted.
We hypothesized that an elevated cell count or differential from specimens obtained from the disc space is associated with NVO.
We obtained approval for this study from our institutional board review and human research ethics committee. This was a prospective proof-of-concept (POC) cohort study of patients with suspected NVO. They were referred to the Division of Neuroradiology for an image-guided aspiration at our institution from 1 January 2019 through 31 December 2019. Patients consented to research participation and gave procedural consent prior to enrollment. The purpose of this study was to establish the optimal white blood cell (WBC) counts and polymorphonuclear leukocyte (PMN) differential cutoffs on specimens obtained from aspirated intervertebral disc space fluid in patients with suspected NVO using POC analysis.
Inclusion criteria for the study included patients 18 years or older with a clinical suspicion of spinal infection and clinical symptoms (back pain) and suspected NVO. Suspected NVO was defined as the presence of back pain, elevated erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP), and MRI findings consistent with NVO (T2 hyperintensity and/or enhancement of the vertebral body sub-endplate regions and/or disc space and/or the presence of paraspinal or epidural inflammation/abscess). We prospectively enrolled patients with a suspected diagnosis of NVO that were referred to the Division of Neuroradiology and Division of Infectious Diseases and other clinical services for image-guided needle aspiration of the intervertebral disc.
The current clinical practice is to send an aspirated specimen from the disc space, obtained via image guidance, to the laboratory for histopathology and cultures. For this study, a manual cell count of the aspirated specimen and a differential were performed using cytospin and touch prep techniques. Image-guided biopsy was performed with an Osteo-Site Murphy co-axial bone biopsy needle set (11- or 13-gauge outer needle, with a 14- or 16-gauge inner trephine needle, respectively), except case 11, in which a 14-gauge Jamshidi (Argon medical device) needle was used. All procedures were performed under local anesthesia.
From the neuroradiology lab, two research specimens were sent to the pathology laboratory.
First, a touch prep slide was prepared using aspirated bone or disc space contents. If two sites were sampled, then two touch prep slides were sent. The second specimen sent was the needle rinse. At the end of routine clinical specimen collection, the operator did a needle rinse with 4 cm3 of normal saline in the neuroradiology lab. In order to standardize volume, we rinsed the needle so that 2 cm3 of fluid was sent to microbiology for routine cultures (clinical specimen), and the remaining 2 cm3 of the needle rinse was sent to the pathology lab for research purposes to run cell counts and differentials. Needle rinse was performed in all cases.
A hematopathologist performed a manual cell count from the aspirate. It was generated by counting the number of cells in a calibrated chamber without staining the aspirate. The volume of aspirate for the manual cell count was 100 µL. The touch prep technique involved dabbing the disc contents/tissue onto the slide to distribute a thin layer of cells for microscopic examination to provide a differential. The cytospin technique involved centrifuging the aspirate, depositing the cells onto the microscopic slide, and providing a differential. The volume of aspirate used for cytospin was 100 µL.
We collected relevant demographic details of patients. Symptomatology and duration of symptoms, history of intravenous drug abuse, laboratory data including WBC count, ESR and CRP, blood cultures, and receipt of antibiotics prior to sampling were recorded. We recorded microbiologic data performed on aspirated specimens, including Gram stain, aerobic and anaerobic bacterial cultures, and 16S ribosomal RNA (rRNA) gene polymerase chain reaction (PCR) (when performed). Routine disc aspirate samples were collected in sterile containers or anaerobic vials (when anaerobic cultures were ordered). Tissue is then processed in a stomacher and then plated on the media (typically, sheep blood agar to isolate gram-positive bacteria and Eosin methylene blue agar for gram-negative bacteria). Aerobic and anaerobic cultures are incubated for 5 and 14 d, respectively.
For statistical analysis, we used JMP statistical software to calculate the receiver operating curve (ROC) based on the presence of infection. NVO was diagnosed based on a positive culture or 16S rRNA gene PCR associated with MRI images consistent with infection. Subset analysis was performed following the elimination of samples due to Cutibacterium acnes. The ROC was used to calculate the cutoff for manual cell count and the neutrophil percentage that would be consistent with infection.
During the enrollment period, 17 aspirates were performed in 14 individual patients. The median age was 70.5 years (range 45–77), and there were six females. The patients presented with back pain with a median duration of 50 d prior to image-guided biopsy (range: 10–173 d). Nine patients (64 %) had received prior antibiotics. Blood cultures were negative in all except for case 12. A total of 12 patients had blood cultures collected 2 or more days prior to image-guided sampling. Two patients had received intra-thecal stem cell injections in the past 2 months before the onset of back pain (cases 6 and 8). None of the patients had a history of intravenous drug abuse.
The median WBC count was 7.55×10 (9) L−1 (range: 5.1–13.2), the median CRP level was 21.25 mg L−1 (range: 3.0–226), and the median ESR was 61 mm h−1 (range: 8–112). The lumbosacral and thoracic spine were involved in 10 and 4 cases, respectively. The specimens were bloody in appearance in four, serosanguinous in nine, and clear in four procedures.
The median manual cell count on the aspirated fluid was 52 cells µL−1 (range: 0–6656). The median neutrophil percentage on the touch prep slide (available in samples as we did not recover cells in four touch prep samples) was 73 % (range: 5 %–100 %), and the median neutrophil percentage on the cytospin slide was 82 % (range: 0 %–100 %). Based on either positive microbiology or a positive 16S rRNA gene PCR of disc aspirates, seven patients (n=8 aspirates) were clinically diagnosed with an infection. For these eight aspirates, the median manual cell count was 260 cells µL−1 (range: 0–6656), and the median neutrophil percentage via cytospin was 92.5 % (range: 58 %–100 %). The median touch prep neutrophil percentage, available in six out of eight aspirated specimens, was 85.5 % (range: 27 %–100 %).
Seven patients (n=9 aspirates) had negative microbiology and/or a negative 16S rRNA gene PCR. The median manual cell count for these nine aspirates was 0 cells µL−1 (range: 0–728), and the median neutrophil percentage via cytospin was 63 % (range: 0 %–88 %). The median touch prep neutrophil percentage was 55 % (range: 5 %–84 %), available in seven out of nine specimens.
Routine bacterial cultures were positive in five cases, and the 16S rRNA gene PCR was positive in two cases (Table 1). The cell count in two patients with Cutibacterium acnes infection generated a lower percentage of neutrophils versus patients with non-Cutibacterium acnes infection.
Table 1Disc specimen manual cell count, differentials, and microbiology of patients with suspected native vertebral osteomyelitis. Cases that were positive for infection are in bold font. CFX is ceftriaxone; DPT is daptomycin; Rif is rifampin; CIPRO is ciprofloxacin; DAIR is debridement, antibiotics, and implant retention; and Vanc is vancomycin. BSI is blood stream infection, and EOT is end of therapy.
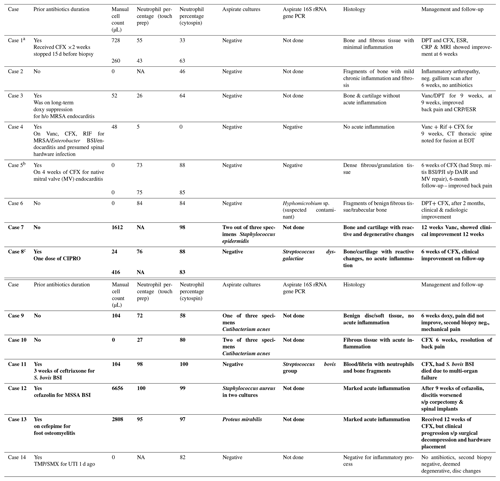
a Case 1: two aspirates. b Case 5: two aspirates. c Case 8: two aspirates.
Using the ROC, we calculated the optimal cutoff for a cell count of 104 total nucleated cells, offering a sensitivity and specificity of 86 % (area under the curve (AUC) of 0.837). A neutrophil percentage cutoff of 83 % was associated with a 71 % sensitivity and specificity (AUC = 0.837, Table 2).
This is the first study to report the utility of cell counts and neutrophil differentials from the aspirated specimen from the intervertebral disc space in patients with suspected NVO. Based on the results of this pilot study, a manual count of 104 total nucleated cells µL−1 or more in aspirated specimens with a neutrophilic predominance (≥83 %) offered the optimal sensitivity and specificity in diagnosing bacterial NVO.
The observation that patients with positive Cutibacterium acnes cultures manifested a lower percentage of neutrophils and low manual cell counts is consistent with previously reported data from other orthopedic infections such as total shoulder arthroplasty (TSA) infection, in which this organism provokes a minimal inflammatory response (Patel et al., 2020). However, data surrounding Cutibacterium acnes TSA are being challenged. In a recent study by Strahm et al. (2018), Cutibacterium acnes was not associated with a low cell count in patients with shoulder arthroplasty infection (Strahm et al., 2018). Therefore, we may be unable to extrapolate data from TSA infection to those with Cutibacterium spinal discitis and osteomyelitis. In a retrospective study of histopathology of perispinal implant tissue samples, infections caused by C. acnes were not commonly associated with inflammatory changes (Burger et al., 2020) as C. acnes is an indolent pathogen and does not cause an intense inflammatory reaction.
Compared to intervertebral disc space infection, the established cutoffs for native joint septic arthritis are much higher. The synovial fluid WBC count for native septic arthritis is typically 50 000 cells mm−3, with a PMN predominance. Cutoff numbers for WBC counts are much lower for diagnosing periprosthetic joint infection (PJI) than native septic arthritis. For hip PJI, the thresholds for WBC and PMN differentials are 4200 WBCs µL−1 and 80 %, respectively, whereas, for knee PJIs, thresholds for WBC and PMNs of 1700 WBCs µL−1 and 65 % PMNs are often used.
We recommend cytospin preparation for differential counts based on several instances (n=4) of touch prep slides where insufficient cells were recovered for differential counts. This also will limit pre-analytic variability caused by varying operators in slide creation and, also, the additional diagnostic specimens.
Given the low sensitivity of image-guided aspirate cultures, providers evaluating patients they clinically suspect have NVO are often faced with uncertainty around the optimal next diagnostic steps. The addition of cell count and differential to aspirated samples from disc space was associated with high sensitivity and specificity in this proof-of-concept study; these markers seem to be elevated in cases of infection compared to cases without a clinical diagnosis of infection. When added to a negative aspirate culture, providers may opt for a watchful approach in patients with imaging suggestive of NVO but a low cell count and neutrophil percentage in disc space samplings.
Alternatively, patients with high cell count or neutrophil percentage and negative cultures (which could be due to prior antibiotics, such as in case 1) may be subjected to either a repeat image-guided sampling or an open biopsy.
Bacterial vertebral osteomyelitis could also have been missed due to sampling errors, as the biopsy is operator-dependent. Thus, it is suggested that patients with initial negative aspirate cultures, histopathology, and low manual cell count or neutrophil differential should have a clinical follow-up to assess the need for a second biopsy if there is a clinical suspicion of discitis and osteomyelitis persists.
Given a limited number of patients, confirmation of applicability to a patient population with back pain and MRI findings due to other conditions, such as patients with spinal malignancy, multiple myeloma, degenerative disc disease, or spinal gout, will be needed in a larger study (Rufener et al., 2012). The study was also not protocolized to run a 16S rRNA gene PCR on all aspirated samples. The physician treating infectious diseases requested this test. In our study, all patients had abnormal MRI findings and elevated inflammatory markers. However, NVO can be detected in patients with normal inflammatory markers, so a more extensive study is needed to examine this subset of patients. We obtained 17 samples from 14 patients (three had two aspirations). This may introduce a statistical bias in the analysis.
In conclusion, as the number of patients with NVO increases, novel methodologies to confirm the diagnosis are warranted. Based on this pilot study, a high manual cell count or a high neutrophilic predominance was associated with the diagnosis of NVO in this pilot study. Since the identified cutoffs in this pilot study were derived from a small number of patients, a subsequent study using a larger cohort is warranted.
| NVO | native vertebral osteomyelitis |
| CRP | C-reactive protein |
| ESR | erythrocyte sedimentation rate |
| PCR | polymerase chain reaction |
| ROC | receiver operating curve |
| TSA | total shoulder arthroplasty |
| PJI | periprosthetic joint infection |
| EOT | end of therapy |
| BSI | bloodstream infection |
| DPT | daptomycin |
| CFX | ceftriaxone |
| CIPRO | ciprofloxacin |
| Rif | rifampin |
| DAIR | debridement, antibiotics, and implant retention |
| Vanc | vancomycin |
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request and after seeking permission from Mayo Clinic.
TR wrote the protocol, obtained consent from the patients, reviewed patient charts, collected data, and contributed to the writing of the manuscript. MH analyzed the pathology specimens and slides and provided data on the cell counts. FD performed procedures on the subjects and contributed to revision of the paper. AJT helped with writing the protocol and statistical analysis. CR helped in recruiting patients and obtaining their consent for the study. PH contributed to the writing and revision of the manuscript. EB helped in writing the protocol and was a major contributor in writing the manuscript.
At least one of the (co-)authors is a member of the editorial board of Journal of Bone and Joint Infection. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Patients consented to research participation and gave procedural consent prior to enrollment. All research participants gave written consent prior to enrollment in this study and for publication. Mayo Clinic human research ethics committee approval was obtained for the purpose of this research.
Preliminary results were presented as an oral presentation at the Musculoskeletal Infection Society meeting (MSIS), August 2019, in NY. The authors confirm that
the manuscript is an honest, accurate, and transparent account of the study
being reported.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The research was supported by the Division of Infectious Diseases, Mayo Clinic, Rochester. Funds were used in conducting the additional research-based tests in the pathology lab for this study.
This paper was edited by Parham Sendi and reviewed by two anonymous referees.
Babic, M. and Simpfendorfer, C. S.: Infections of the Spine, Infect. Dis. Clin. North Am., 31, 279–297, https://doi.org/10.1016/j.idc.2017.01.003, 2017.
Berbari, E. F., Kanj, S. S., Kowalski, T. J., Darouiche, R. O., Widmer, A. F., Schmitt, S. K., Hendershot, E. F., Holtom, P. D., Huddleston III, P. M., Petermann, G. W., and Osmon, D. R.: Executive Summary: 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults, Clin. Infect. Dis., 61, 859–863, https://doi.org/10.1093/cid/civ633, 2015.
Burger, J., Palmowski, Y., Strube, P., Perka, C., Putzier, M., and Pumberger, M.: Low sensitivity of histopathological examination of peri-implant tissue samples in diagnosing postoperative spinal implant infection, Bone Joint J., 102-B, 899–903, https://doi.org/10.1302/0301-620X.102B7.BJJ-2019-1725.R2, 2020.
Colmenero, J. D., Ruiz-Mesa, J. D., Sanjuan-Jimenez, R., Sobrino, B., and Morata, P.: Establishing the diagnosis of tuberculous vertebral osteomyelitis, Eur. Spine J., 22 Suppl 4, 579–586, https://doi.org/10.1007/s00586-012-2348-2, 2013.
Czuczman, G. J., Marrero, D. E., Huang, A. J., Mandell, J. C., Ghazikhanian, V., and Simeone, F. J.: Diagnostic yield of repeat CT-guided biopsy for suspected infectious spondylodiscitis, Skeletal Radiol., 47, 1403–1410, https://doi.org/10.1007/s00256-018-2972-y, 2018.
Eren Gok, S., Kaptanoglu, E., Celikbas, A., Ergonul, O., Baykam, N., Eroglu, M., and Dokuzoguz, B.: Vertebral osteomyelitis: clinical features and diagnosis, Clin. Microbiol. Infect., 20, 1055–1060, https://doi.org/10.1111/1469-0691.12653, 2014.
Fragio Gil, J. J., Gonzalez Mazario, R., Ivorra Cortes, J., Canada Martinez, A. J., Salavert Lleti, M., and Roman Ivorra, J. A.: CT-Guided Needle Biopsy in Vertebral Osteomyelitis: Study of Factors That Could Influence in Culture Yield, Reumatol. Clin. (Engl. Ed)., 18, 20–24, https://doi.org/10.1016/j.reumae.2020.08.007, 2022.
Issa, K., Diebo, B. G., Faloon, M., Naziri, Q., Pourtaheri, S., Paulino, C. B., and Emami, A.: The Epidemiology of Vertebral Osteomyelitis in the United States From 1998 to 2013, Clin. Spine Surg., 31, E102–E108, https://doi.org/10.1097/BSD.0000000000000597, 2018.
Kremers, H. M., Nwojo, M. E., Ransom, J. E., Wood-Wentz, C. M., Melton III, L. J., and Huddleston III, P. M.: Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009, J. Bone Joint Surg. Am., 97, 837–845, https://doi.org/10.2106/JBJS.N.01350, 2015.
Mylona, E., Samarkos, M., Kakalou, E., Fanourgiakis, P., and Skoutelis, A.: Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics, Semin. Arthritis Rheum., 39, 10–1, https://doi.org/10.1016/j.semarthrit.2008.03.002, 2009.
Nickerson, E. K. and Sinha, R.: Vertebral osteomyelitis in adults: an update, Br. Med. Bull., 117, 121–138, https://doi.org/10.1093/bmb/ldw003, 2016.
Patel, M. S., Singh, A. M., Gregori, P., Horneff, J. G., Namdari, S., and Lazarus, M. D.: Cutibacterium acnes: a threat to shoulder surgery or an orthopedic red herring?, J. Shoulder Elbow Surg., 29, 1920–1927, https://doi.org/10.1016/j.jse.2020.02.020, 2020.
Rankine, J. J., Barron, D. A., Robinson, P., Millner, P. A., and Dickson, R. A. Therapeutic impact of percutaneous spinal biopsy in spinal infection, Postgrad. Med. J., 80, 607–609, https://doi.org/10.1136/pgmj.2003.017863, 2004.
Rufener, J., Schulze, C. C., Tanzler, K., Aeberli, D., and Sendi, P.: Sterile spondylodiscitis, Lancet, 379, 1850, https://doi.org/10.1016/S0140-6736(11)61924-7, 2012.
Shaltot, A., Michell, P. A., Betts, J. A., Darby, A. J., and Gishen, P.: Jamshidi needle biopsy of bone lesions, Clin. Radiol., 33, 193–196, https://doi.org/10.1016/s0009-9260(82)80061-5, 1982.
Strahm, C., Zdravkovic, V., Egidy, C., and Jost, B.: Accuracy of Synovial Leukocyte and Polymorphonuclear Cell Count in Patients with Shoulder Prosthetic Joint Infection, J. Bone Joint Infect., 3, 245–248, https://doi.org/10.7150/jbji.29289, 2018.
Wong, H., Tarr, G. P., Rajpal, K., Sweetman, L., and Doyle, A.: The impact of antibiotic pre-treatment on diagnostic yield of CT-guided biopsy for spondylodiscitis: A multi-centre retrospective study and meta-analysis, J. Med. Imaging Radiat. Oncol., 65, 146–151, https://doi.org/10.1111/1754-9485.13118, 2021.
Zimmerli, W.: Clinical practice. Vertebral osteomyelitis, N. Engl. J. Med., 362, 1022–1029, https://doi.org/10.1056/NEJMcp0910753, 2010.





