the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Proteus-species-associated periprosthetic hip and knee joint infections – a 15-year cohort analysis
Veronika Achatz
Jennyfer A. Mitterer
Stephanie Huber
Ece Akcicek
Selma Tobudic
Sujeesh Sebastian
Introduction: While Gram-negative periprosthetic joint infections (PJIs) are generally known for their poor outcome, few data on Proteus species exist. Therefore, we investigated the prevalence, clinical characteristics, microbial spectrum, outcomes, antimicrobial treatment, and surgical procedures of Proteus-species-associated PJIs. Methods: We retrospectively evaluated 1776 culture-positive revision hip and knee arthroplasties (hereafter rTHA and rTKA, respectively) from a single institution between 2008 and 2024. The European Bone and Joint Infection Society and International Consensus Meeting criteria were used for classification. The Charlson comorbidity score and tier classification were used for evaluating risk factors and success and failure rates. Statistical analysis was performed using the chi-square test and binary logistic regression. Results: Among 1776 culture-positive revision arthroplasties, we identified 26 (1.5 %) Proteus-species-associated PJIs. The majority were observed in rTHA, mostly in chronic (65.4 %) and polymicrobial (57.7 %) infections. Chronic PJIs were associated with polymicrobial infections (p=0.027), resulting in a higher failure rate (p=0.041). Among polymicrobial infections (15 of 26 cases), Enterococcus faecalis (5 of 15), Staphylococcus epidermidis (4 of 15), and Pseudomonas aeruginosa (3 of 15) were most frequently observed. The most frequently used surgical approach was a two-stage revision (46.2 %), with a success rate of 25 % (3 of 12). Proteus-species-associated PJIs were mainly treated with fluoroquinolone, especially ciprofloxacin showed higher success rates (p=0.018). The reinfection-free survival rate was 48.5 % after 12 months and 22.6 % after 40 months. Conclusion: Proteus species represent a rare group of pathogens and are predominantly found in chronic and polymicrobial PJIs, with a higher occurrence in rTHA than rTKA. Despite an overall high clinical failure rate, ciprofloxacin showed promising antimicrobial treatment efficacy.
- Article
(819 KB) - Full-text XML
-
Supplement
(668 KB) - BibTeX
- EndNote
Periprosthetic joint infections (PJIs) are among the most severe complications of total joint arthroplasty (TJA). While PJIs are predominantly caused by Gram-positive (GP) pathogens, Gram-negative (GN) bacteria are increasingly being reported as causing PJIs (up to 23 % of cases) (Benito et al., 2016; Hsieh et al., 2009; Sebastian et al., 2019). Although GN PJIs are associated with poor outcomes, most studies have focused on GP pathogens; thus, data on GN-pathogen-associated PJIs remain limited (Aboltins et al., 2011; Tande and Patel, 2014; Uçkay and Bernard, 2010).
Pseudomonas (20 %–36 %) and E. coli (3 %–30 %) are the most common pathogens among GN PJI, whereas Proteus spp. only comprise 3 %–15 % of cases (Hsieh et al., 2009; Rodríguez-Pardo et al., 2014; Zmistowski et al., 2011). Proteus spp. are GN opportunistic pathogens known for their clinical manifestation in urinary tract infections (UTIs) and catheter-associated urinary tract infections (CAUTIs) (Schaffer and Pearson, 2015). While recent data indicate a growing number of infections caused by Proteus, little is known about Proteus-species-associated PJI (Armbruster et al., 2018; Facciolà et al., 2022).
Recent studies have proposed that outcomes and treatments differ greatly among GN bacteria, necessitating pathogen-specific therapies (Gonzalez et al., 2024; Uçkay and Bernard, 2010). Given the increasing incidence of GN PJIs and their unique challenges, pathogen-specific studies are required to better understand and manage infections caused by GN organisms. Additionally, the growing concern of antimicrobial resistance among Proteus isolates further complicates the management of these infections and may necessitate extended antimicrobial therapy and multiple surgical interventions.
Therefore, this study evaluated the frequency, clinical characteristics, microbial spectrum, surgical procedures, antibiotic treatment, and outcome of total hip and knee arthroplasty revisions (hereafter rTHA and rTKA, respectively) associated with Proteus spp.
After receiving institutional ethics board approval (EK 10/2020; blinded for review), we investigated 1776 culture-positive knee and hip revision arthroplasties from January 2008 until June 2024 from our prospectively maintained in-house arthroplasty registry and PJI database. All patients who had a positive intraoperative culture for Proteus spp. were included in this study. For PJI classification, the International Consensus Meeting (ICM) 2018 (Shohat et al., 2019) and the European Bone and Joint Infection Society (EBJIS; McNally et al., 2021) criteria were used. PJI cases were classified as acute if the onset occurred within 3 months or as chronic if the onset was longer than 3 months after the primary implantation (Li et al., 2018). Patient specific risk factors were assessed, using the McPherson classification (Coughlan and Taylor, 2020) and the Charlson comorbidity index (CCI; Charlson et al., 1987).
2.1 Microbiological analysis
For microbiological analysis, the following samples were utilized: preoperatively collected synovial fluid, periprosthetic tissue samples, intraoperative swabs, and sonication fluid. Swabs were accepted as positive cultures only when collected intraoperatively. The median number of samples taken per surgery was five, with a median number of four positive intraoperative cultures. Explanted devices were immediately placed into sonication containers, into which saline solution was added to completely cover the implants. The container was then sonicated and vortexed (Trampuz et al., 2007). Tissue samples and sonication fluid (0.1 mL) were further analysed for bacterial and fungal identification using standard microbiological techniques (Frank et al., 2021). Antimicrobial susceptibility profiling was determined using the BD system (Becton Dickinson and Company, Franklin Lakes, NJ), according to the manufacturer's recommendations, and interpreted following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (EUCAST, 2024).
Antibiotic treatment was initially given according to the institutional protocols and subsequently adjusted based on the antibiogram results. Antibiotic resistance was evaluated in all patients. All patients received antimicrobial treatment consisting of at least two antimicrobial agents in combination.
2.2 Follow-up and clinical outcome
Four patients died during the follow-up period. Patients who had treatment failure within 1 year were included, although they did not achieve a 1-year follow-up. If additional information on the follow-up was needed, patients were contacted by phone. Detailed demographic data are displayed in Table 1.
The treatment failure and success rates were calculated using the tier classification by the Musculoskeletal Infection Society (Fillingham et al., 2019). Cases classified in the first group of the tier classification (Tier 1) were considered successful, as the infection was successfully eradicated without further antibiotic treatment. We also included spacer implantation followed by successful reimplantation and complete infection control in the first category. Tier 2 includes patients on suppressive antibiotic therapy. Tier 3 consists of all patients with following surgery and is divided into different subcategories: A – aseptic revision after 1 year; B – septic revision after 1 year; C – aseptic revision within 1 year; D – septic revision within 1 year; E – amputation, resection arthroplasty, and arthrodesis; F – retained spacer. Tier 4 includes all of the patients who either died within 1 year (A) or after 1 year (B).
2.3 Statistical analysis
Demographic variables are presented as means with standard deviations. The body mass index (BMI) and CCI are shown as the mean, standard deviation, and interquartile range (IQR) for age, calculated for all patients. Categorical variables were compared using the chi-square test, while continuous variables were analysed using the Mann–Whitney U test or the t test for normally distributed values. Results were accepted as statistically significant at a p value < 0.05. Statistical analyses were performed using IBM SPSS Statistics version 26 (SPSS Inc, Chicago, IL, USA).
Table 1Demographic data of the study population.
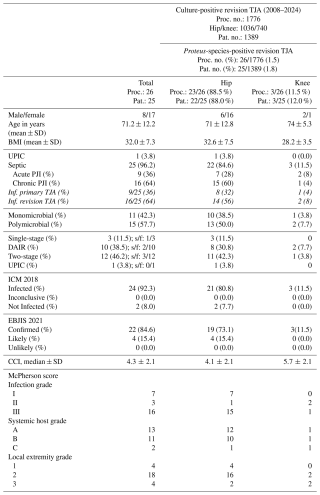
Abbreviations/acronyms used in the table are as follows: TJA – total joint arthroplasty; SD – standard deviation; Proc. no. – procedure number; Pat. no. – patient number; Inf. – infected; UPIC – unexpected positive intraoperative culture; PJI – periprosthetic joint infection; ICM – International Consensus Meeting; EBJIS – European Bone and Joint Infection Society; CCI – Charlson comorbidity index; DAIR – debridement antibiotic, and implant retention; s/f – success/failure. Note: one patient received different surgeries consecutively, counted as one patient in DAIR and one in two-stage procedures.
Out of 1776 culture-positive revision arthroplasties, 26 of 1776 (1.5 %) revision surgeries showed intraoperatively positive results for Proteus spp. These involved 25 patients (8 male and 17 female) who underwent hip (23 of 26, 88.5 %) and knee (3 of 26, 11.5 %) revision surgeries. One case (1 of 26, 3.8 %) was a presumed aseptic rTHA with an unexpectedly positive intraoperative culture (UPIC). Using the ICM criteria, 24 cases were defined as infections, and 2 were identified as not infected, whereas according to EBJIS criteria, 22 cases were identified as infections, and 4 were considered likely to have an infection. The mean age was 71.2 years (IQR: 78.8–68), and the mean BMI (kg m−2) was 32.0 ± 7.3. No statistically significant difference between BMI, CCI, age, and sex between rTHA and rTKA was found. The median follow-up period was 26.9 months (IQR: 5.8–40.6).
3.1 Microbial analysis and polymicrobial infections
In total, 132 samples were collected from 26 revision procedures; of these 104 (78.8 %) had a positive intraoperative culture. Overall, a total of 143 microorganisms were identified, including 80 (55.9 %) cases of Proteus spp. and 63 (44.1 %) cases of other pathogens. Monomicrobial infections with Proteus mirabilis were identified in 11 (42.3 %) of the 26 surgeries. Polymicrobial infections were found in 15 (57.7 %) of the 26 procedures, with P. mirabilis identified in 14 (93.3 %) cases and Proteus vulgaris in 1 (6.7 %) case. Moreover, polymicrobial infections were more frequently observed in patients with chronic PJI (p=0.027), especially those with chronic hip PJI (p=0.02). The same effect could not be shown in knee PJI (p=0.667). Distributions of the microbiological spectrum in rTKA and rTHA are displayed in Table 2.
Table 2Detailed microbiological spectrum in PJI associated with Proteus spp. and categorized in hip and knee infections.
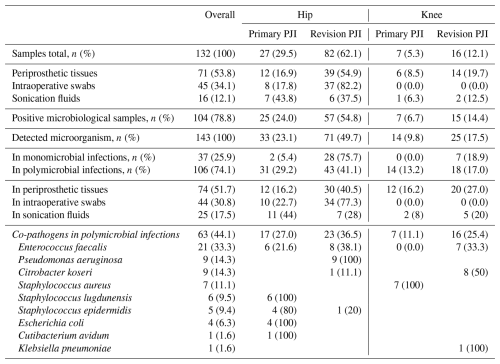
The most common combinations were between Proteus spp. and Enterococcus faecalis in 5 of 15 cases (33.3 %), Staphylococcus epidermidis in 4 of 15 cases (26.7 %), and Pseudomonas aeruginosa in 3 of 15 cases (20 %). The total number of microbial samples (n=63) detected in polymicrobial infections and their distribution in rTHA and rTKA are shown in Table 2. There were combinations of Proteus spp. with up to four microorganisms taken from one joint. The detailed combinations of pathogens in polymicrobial infections are shown in Table 3.
Table 3Detailed microbial profiles, surgical details, antibiotic therapy against Proteus spp., and success/failure rate as per the tier classification. Every line represents one patient. Patients 23 and 24 refer to the same patient but are mentioned separately because they underwent different surgeries and treatments. Antibiotic agents targeting co-pathogens are not mentioned in this table. Please see the Supplement (Table S1) for additional details on antibiotic therapies in polymicrobial infections.

a Antibiotic therapy was given as perioperative prophylaxis. b Antibiotic therapy started before surgery and continued during surgery. c Empirical antibiotic therapy, which was started during surgery and changed accordingly to the antibiogram. d Antibiotic changes classified as continued, switched, de-/escalated, or salvage therapy. Only agents targeting Proteus spp. are shown. Abbreviations/acronyms used in the table are as follows: Pat. no. – patient number; PoD – postoperative day; rTHA – revision total hip arthroplasty; rTKA – revision total knee arthroplasty; DAIR – debridement antibiotic, and implant retention; UPIC – unexpected, positive intraoperative culture; PIT – piperacillin–tazobactam; TRS – trimethoprim–sulfametrole; AMC – amoxicillin/clavulanic acid. Tier classifications are as follows: 1 – successful without suppressive antibiotic therapy; 3B – septic revision >1 year after PJI diagnosis; 3D – septic revision ≤1 year after PJI treatment; 3E – amputation, resection arthroplasty, and arthrodesis; 3F – retained spacer; 4A – death within 1 year of PJI treatment.
3.2 Resistance pattern and antimicrobial treatment
Antibiograms showed resistance against amoxicillin clavulanic acid (in 3 of 25 cases, 12 %), ampicillin (in 7 of 19 cases, 36.8 %), ciprofloxacin (in 5 of 25 cases, 20 %), gentamicin (in 3 of 22 cases, 13.6 %), fosfomycin (in 3 of 15 cases, 20 %), piperacillin (in 2 of 17 cases, 11.8 %), tobramycin (in 5 of 17 cases, 29.4 %), amikacin (in 1 of 22 cases, 4.5 %), ceftazidime (in 1 of 22 cases, 4.5 %), cefuroxime (in 7 of 23 cases, 30.4 %), cefepime (in 1 of 20 cases, 5 %), and trimethoprim–sulfamethoxazole (in 5 of 20 cases, 25 %). With respect to fluoroquinolones, ciprofloxacin showed resistance in 5 out of 25 tested samples (20 %), moxifloxacin showed resistance in 1 out of 3 tested samples (33.3 %), and levofloxacin showed resistance in 5 out of 22 tested samples (22.7 %). Antibiotic resistance patterns are shown in Fig. 1.
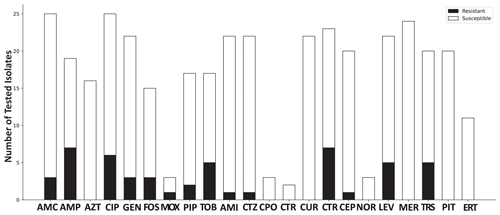
Figure 1Antibiotic resistance pattern, giving the total number of tested isolates categorized as resistant and susceptible. Abbreviations used in the figure are as follows: AMC – amoxicillin/clavulanic acid; AMP – ampicillin; AZT – aztreonam; CIP – ciprofloxacin; GEN – gentamicin; FOS – fosfomycin; MOX – moxifloxacin; PIP – piperacillin; TOB – tobramycin; AMI – amikacin; CTZ – ceftazidime; CIX – cefixime; CPO – cefpodoxime; CTR – ceftriaxone; CUR – cefuroxime; CEP – cefepime; NOR – norfloxacin; LEV – levofloxacin; MER – meropenem; TRS – trimethoprim–sulfamethoxazole; PIT – piperacillin–tazobactam; ERT – ertapenem.
Perioperatively, cefuroxime alone or in combination with other antibiotics was administered in 7 out of 26 patients. Teicoplanin was given to 9 out of 26 patients during surgery and had already been started in 6 out of 9 patients preoperatively because of previous GP infections in some cases and continued during surgery. The remaining patients received various other antibiotics as empirical therapy. Detailed antibiotic treatments against Proteus spp. are presented in Table 3, and antibiotic therapies targeting other pathogens are summarized in Table S1 in the Supplement. Empiric therapy was then de-escalated according to the resistance pattern. The antimicrobial treatment was given for a mean of 14.4 ± 7.5 weeks. A fluoroquinolone was administered in 18 out of 26 cases, with ciprofloxacin used in 10 cases (36 %) and moxifloxacin in 6 cases (23.1 %). In 10 of 26 cases (36 %), ciprofloxacin alone or combined with other antibiotics was given. Ciprofloxacin administration showed a significant association with a success rate of 5 out of 10 cases (50 %; p=0.018). However, no statistically significant association between all fluoroquinolones and the success rate could be identified (p=0.292).
3.3 Surgical procedures and outcomes
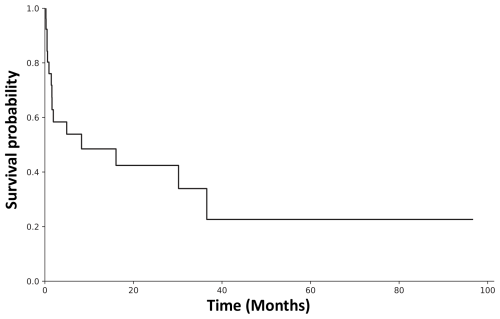
Overall, in the 26 patients, 9 (34.6 %) infected primary TJA and 17 (65.4 %) infected revision TJAs were identified. Infected revision TJAs were primarily associated with persistent infections in 14 of 17 cases (82.4 %), with other microorganisms identified in prior septic revisions. In the case of chronic infections of revision TJAs, a higher failure rate was observed (p=0.041) compared to acute infections. Polymicrobial and monomicrobial infections did not significantly correlate with the success or failure rate (p=0.612). Revisions performed at culture sampling were debridement antibiotic, and implant retention (DAIR) in 10 of 26 cases (38.5 %); two-stage revisions in 12 of 26 cases (46.2 %; spacer in 9 of the 12 cases and resection arthroplasty in 3 of the 12 cases); septic single-stage revision in 3 of 26 cases (11.5 %); and presumed aseptic single-stage revision in 1 of 26 cases (3.8 %). Differences in success rates depending on the surgical procedure were not observed (p=0.579), with overall success in 6 of 26 cases (23.1 %). The highest success rate of 1 of 3 cases (33.3 %) was observed in single-stage procedures, whereas the success rate in two-stage procedures was 3 of 12 cases (25.0 %). In contrast, DAIR procedures were related to septic failure in 8 of 10 cases (80.0 %). Additionally, the UPIC case resulted in a septic failure in the consecutive re-revision procedure. The overall reinfection rate is illustrated in Fig. 2. Infection-free survival after 12 months was 48.5 %, whereas it was 22.6 % after 40 months.
In this study, Proteus pathogen was identified in 26 of 1776 cases (1.5 %) in rTHAs and rTKAs. Notably, Proteus-species-associated PJIs were observed to be more prevalent in rTHAs (88.5 %) compared to rTKAs (11.5 %). Furthermore, the majority of the Proteus-species-associated PJIs were identified in polymicrobial infections (15 of 26 cases, 57.7 %) and chronic revisions (16 of 25 cases, 64 %), resulting in a high failure rate.
The prevalence of Proteus spp. in 1.5 % of rTHA and rTKA surgeries observed in our study was lower compared with the respective values of 4.8 % and 2.6 % reported by Benito et al. (2016) and van Veghel et al. (2024). Moreover, the higher incidence of Proteus-species-associated PJI in rTHA (88.5 %) than in rTKA (11.5 %) is corroborated by van Veghel et al. (2024). Polymicrobial infections were more prevalent in chronic rTHA (p=0.02) than in chronic rTKA (p=0.667), with Enterococcus spp., Pseudomonas spp., and S. epidermidis being the most frequent pathogens. Enterococcus spp. and E. coli as co-pathogens were especially prevalent in rTHA, mainly due to the hip's unique anatomical and microbiological environment and gut colonization of Enterococcus spp., which could lead to contamination during or after surgery (Chisari et al., 2022; Mitterer et al., 2024). The reason for the more frequent association of Proteus with rTHA compared to rTKA remains unknown. However, Loewik et al. (2019) demonstrated a significantly higher prevalence of Proteus spp. in rTHA in extremely obese patients compared to rTKA, which is consistent with our findings. Although the association of obesity and Proteus spp. infections was not investigated in our cohort, compared to other cohorts, the mean BMI in our cohort was >30 kg m−2, which could have influenced the risk of Proteus infections. While Proteus is known for its common colonization of the urogenital tract and its association with UTIs, recent studies have identified a correlation between UTIs and PJIs (Blanchard et al., 2022). However, the present study could not identify any association between UTIs and Proteus-species-associated PJIs.
Notably, persistent infections with Proteus spp. were rare, as only one patient presented with a Proteus infection in two consecutive surgeries. This may be attributed to the frequent changes in microorganisms throughout revision surgeries and their association with polymicrobial infections, making their detection more difficult (Frank et al., 2021; McCulloch et al., 2023). As previous studies have shown, changes in the microbial spectrum throughout revision surgeries are not necessarily considered to be new infections but, rather, infections that have not been previously detected (Frank et al., 2021). This study showed that polymicrobial infections were especially found in chronic revisions, as corroborated by previous findings (Li et al., 2021). Additionally, studies found that the number of pathogens increases with the number of revisions, leading to a higher occurrence of polymicrobial infections in chronic PJI (McCulloch et al., 2023). Kavolus et al. (2019) recently proved that polymicrobial infections have poorer outcomes. In congruence with their findings, we also observed a low success rate of Proteus-species-associated polymicrobial PJIs.
The most common antibiotic treatment in our study included fluoroquinolones, a recommended treatment for Proteus spp. infections (Osmon et al., 2012) due to their biofilm penetration (Przekwas et al., 2022) and ability to achieve effective therapeutic concentrations in tissue as well as bone penetration (Landersdorfer et al., 2009). This was evident, as PJIs treated with ciprofloxacin had a higher success rate. Previous studies have also shown a better outcome in patients with GN PJI treated with ciprofloxacin (Martínez-Pastor et al., 2009). However, Proteus isolates in our study showed resistance to ciprofloxacin in 20 % of the tested samples, resulting in treatment failures. Kwiecinska-Piróg et al. (2013) reported even higher resistance of Proteus spp. isolates to ciprofloxacin (40 %, or 20 of 50 cases) (2013).
Two-stage procedures are recommended for GN and chronic PJI (Hsieh et al., 2009; Kildow et al., 2022). In this study, most interventions were two-stage revisions due to the chronic nature of the PJIs. Two-stage and one-stage revisions had the highest success rate, up to one-third of all cases, resulting in infection control. The low number of DAIR procedures is because most of the Proteus-species-associated PJIs were chronic PJIs. Previous studies showed that DAIR procedures are not recommended in chronic revision cases or GN-associated infections (Zhu et al., 2021). However, due to the small number of surgical procedures, no recommendation for a surgical procedure can be made.
This study has limitations due to its retrospective nature and the small number of Proteus-species-associated PJIs identified, resulting in a heterogeneous analysis. Moreover, the number of sample acquisitions differed greatly depending on the surgeon's preference, and tissue extraction was not standardized earlier. Consequently, many swabs were accepted as intraoperative cultures, although they were not tissue cultures. This discrepancy may result in differences and inaccuracies in microbial analysis. Furthermore, this work was a single-centre study, resulting in differences in PJI etiology and antibiotic resistance compared to other institutions. However, to our knowledge, no prior studies have focused on Proteus-species-associated PJIs in this context, making direct comparisons challenging.
In conclusion, Proteus spp. present significant challenges in revision arthroplasty, as they mainly occur in polymicrobial and chronic revision PJIs, with a higher prevalence in hip PJIs. Treatment options for chronic and polymicrobial infections are limited and make it difficult to carry out successful treatment. Although fluoroquinolones, especially ciprofloxacin, showed a promising antimicrobial treatment, the growing resistance is concerning. Future studies are required to develop pathogen-specific strategies for optimal treatment of these cases.
Data can be provided upon reasonable request.
The supplement related to this article is available online at https://doi.org/10.5194/jbji-10-265-2025-supplement.
VA: methodology, formal analysis, investigation, data curation, and writing – original draft preparation; JM: data curation and writing – review and editing; SH: data curation and writing – review and editing; EA: writing – review and editing; ST: writing – review and editing; SuS: writing – review and editing; JGH: conceptualization, supervision, and writing – review and editing. All authors critically revised the manuscript and accepted the final version for publication.
The contact author has declared that none of the authors has any competing interests.
The study was conducted according to the principles of the Declaration of Helsinki and its later amendments. The study was approved by the institutional review board of the ethics committee of the Vinzenz group (EK 10/2020).
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This paper was edited by Alex Soriano Viladomiu and reviewed by two anonymous referees.
Aboltins, C. A., Dowsey, M. M., Buising, K. L., Peel, T. N., Daffy, J. R., Choong, P. F. M., and Stanley, P. A.: Gram-negative prosthetic joint infection treated with debridement, prosthesis retention and antibiotic regimens including a fluoroquinolone, Clin. Microbiol. Infec., 17, 862–867, https://doi.org/10.1111/j.1469-0691.2010.03361.x, 2011.
Armbruster, C. E., Mobley, H. L. T., and Pearson, M. M.: Pathogenesis of Proteus mirabilis Infection, EcoSal Plus, 8, https://doi.org/10.1128/ecosalplus.ESP-0009-2017, 2018.
Benito, N., Franco, M., Ribera, A., Soriano, A., Rodriguez-Pardo, D., Sorlí, L., Fresco, G., Fernández-Sampedro, M., Dolores del Toro, M., Guío, L., Sánchez-Rivas, E., Bahamonde, A., Riera, M., Esteban, J., Baraia-Etxaburu, J. M., Martínez-Alvarez, J., Jover-Sáenz, A., Dueñas, C., Ramos, A., Sobrino, B., Euba, G., Morata, L., Pigrau, C., Coll, P., Mur, I., Ariza, J., Barcenilla, F., Pérez-Villar, F., Prats-Gispert, L., Cisterna, R., Ibarra, S., López, Santamaría, J. M., Cabo, J., García, D., Lora-Tamayo, J., Murillo, O., Pedrero, S., Álvarez-Parrondo, S., Muedra-Font, R., Raya-Fernández, C., Rodríguez-Alonso, C., Moreno, A., Blanco-Martínez-de-Morentin, M. A., Cabo-Magadan, R., Combalia, A., García, S., Martínez-Pastor, J. C., Tornero, E., Merino-Pérez, J., Montejo, J. M., Alier, A., Horcajada, J. P., Plasencia, V., Puig, L., Auñon, Blanco, A., García-Cañete, J., Sandoval, E., Fakkas-Fernández, M., Garcés-Zarzalejo, C., Fariñas-Alvarez, C., Fariñas, M. C., Martinez-Martinez, L., Salas-Venero, C., Cobo, J., Ruiz-Carbajosa, P., Jordán, M., Crusi, X., Marinescu, C., Montaner, F., Ramírez, A., Corona, P. S., Lung, M., Muniain-Ezcurra, M., Peñas-Espinar, C., Suárez, A. I., Álvarez, R., Cordero, J. A., López-Pliego, M., Palomino, J., and Puente, A.: Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study, Clin. Microbiol. Infec., 22, 732.e1–732.e8, https://doi.org/10.1016/j.cmi.2016.05.004, 2016.
Blanchard, N. P., Browne, J. A., and Werner, B. C.: The Timing of Preoperative Urinary Tract Infection Influences the Risk of Prosthetic Joint Infection Following Primary Total Hip and Knee Arthroplasty, J. Arthroplasty, 37, 2251–2256, https://doi.org/10.1016/j.arth.2022.05.034, 2022.
Charlson, M. E., Pompei, P., Ales, K. L., and MacKenzie, C. R.: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation, J. Chron. Dis., 40, 373–383, https://doi.org/10.1016/0021-9681(87)90171-8, 1987.
Chisari, E., Cho, J., Wouthuyzen-Bakker, M., and Parvizi, J.: Periprosthetic Joint Infection and the Trojan Horse Theory: Examining the Role of Gut Dysbiosis and Epithelial Integrity, J. Arthroplasty, 37, 1369–1374, https://doi.org/10.1016/j.arth.2022.03.030, 2022.
Coughlan, A. and Taylor, F.: Classifications in Brief: The McPherson Classification of Periprosthetic Infection, Clin. Orthop. Relat. R., 478, 903–908, https://doi.org/10.1097/CORR.0000000000001133, 2020.
EUCAST: Clinical breakpoints and dosing of antibiotics, https://www.eucast.org/clinical_breakpoints, last access: 18 December 2024.
Facciolà, A., Gioffrè, M. E., Chiera, D., Ferlazzo, M., Virga, A., and Laganà, P.: Evaluation of antibiotic resistance in Proteus spp: a growing trend that worries Public Health. Results of 10 Years of Analysis, New Microbiol., 45, 269–277, 2022.
Fillingham, Y. A., Della Valle, C. J., Suleiman, L. I., Springer, B. D., Gehrke, T., Bini, S. A., Segreti, J., Chen, A. F., Goswami, K., Tan, T. L., Shohat, N., Diaz-Ledezma, C., Schwartz, A. J., and Parvizi, J.: Definition of Successful Infection Management and Guidelines for Reporting of Outcomes after Surgical Treatment of Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society (MSIS), J. Bone Joint Surg., 101, e69, https://doi.org/10.2106/JBJS.19.00062, 2019.
Frank, B. J. H., Aichmair, A., Simon, S., Schwarz, G. M., Dominkus, M., and Hofstaetter, J. G.: Analysis of Culture Positive First and Second Stage Procedures in Periprosthetic Knee and Hip Joint Infections, J. Arthroplasty, 36, 2158–2164, https://doi.org/10.1016/j.arth.2021.01.074, 2021.
Gonzalez, M. R., Gonzalez, J., Patel, R. V., Werenski, J. O., Lizcano, J. D., and Lozano-Calderon, S. A.: Microbiology, Treatment, and Postoperative Outcomes of Gram-Negative Prosthetic Joint Infections – A Systematic Review of the Literature, J. Am. Acad. Orthop. Sur., 33, e327–e339, https://doi.org/10.5435/JAAOS-D-23-01203, 2024.
Hsieh, P.-H., Lee, M. S., Hsu, K.-Y., Chang, Y.-H., Shih, H.-N., and Ueng, S. W.: Gram-Negative Prosthetic Joint Infections: Risk Factors and Outcome of Treatment, Clin. Infect. Dis., 49, 1036–1043, https://doi.org/10.1086/605593, 2009.
Kavolus, J. J., Cunningham, D. J., Rao, S. R., Wellman, S. S., and Seyler, T. M.: Polymicrobial Infections in Hip Arthroplasty: Lower Treatment Success Rate, Increased Surgery, and Longer Hospitalization, J. Arthroplasty, 34, 710–716, https://doi.org/10.1016/j.arth.2018.09.090, 2019.
Kildow, B. J., Springer, B. D., Brown, T. S., Lyden, E., Fehring, T. K., and Garvin, K. L.: Long Term Results of Two-Stage Revision for Chronic Periprosthetic Hip Infection: A Multicenter Study, J. Clin. Med., 11, 1657, https://doi.org/10.3390/jcm11061657, 2022.
Kwiecinska-Piróg, J., Skowron, K., Zniszczol, K., and Gospodarek, E.: The Assessment of Proteus mirabilis Susceptibility to Ceftazidime and Ciprofloxacin and the Impact of These Antibiotics at Subinhibitory Concentrations on Proteus mirabilis Biofilms, Biomed. Res. Int., 2013, 930876, https://doi.org/10.1155/2013/930876, 2013.
Landersdorfer, C. B., Rgen, J., Bulitta, B., Kinzig, M., Holzgrabe, U., and Sörgel, F.: Penetration of Antibacterials into Bone Pharmacokinetic, Pharmacodynamic and Bioanalytical Considerations, Clin. Pharmacokinet., 48, 89–124, https://doi.org/10.2165/00003088-200948020-00002, 2009.
Li, C., Renz, N., and Trampuz, A.: Management of Periprosthetic Joint Infection, Hip Pelvis, 30, 138–146, https://doi.org/10.5371/hp.2018.30.3.138, 2018.
Li, H., Fu, J., Niu, E., Chai, W., Xu, C., Hao, L. B., and Chen, J.: The risk factors of polymicrobial periprosthetic joint infection: a single-center retrospective cohort study, BMC Musculoskelet. Di., 22, 1–7, https://doi.org/10.1186/S12891-021-04664-0, 2021.
Loewik, C. A. M., Zijlstra, W. P., Knobben, B. A. S., Ploegmakers, J. J. W., Dijkstra, B., De Vries, A. J., Kampinga, G. A., Mithoe, G., Al Moujahid, A., Jutte, P. C., and Wouthuyzen-Bakker, M.: Obese patients have higher rates of polymicrobial and Gram-negative early periprosthetic joint infections of the hip than non-obese patients, PLoS One, 14, e0215035, https://doi.org/10.1371/journal.pone.0215035, 2019.
Martínez-Pastor, J. C., Muñoz-Mahamud, E., Vilchez, F., García-Ramiro, S., Bori, G., Sierra, J., Martínez, J. A., Font, L., Mensa, J., and Soriano, A.: Outcome of acute prosthetic joint infections due to gram-negative bacilli treated with open debridement and retention of the prosthesis, Antimicrob. Agents Chemother., 53, 4772–4777, https://doi.org/10.1128/AAC.00188-09, 2009.
McCulloch, R. A., Martin, A., Young, B. C., Kendrick, B. J., Alvand, A., Jeys, L., Stevenson, J., and Palmer, A. J.: Frequent microbiological profile changes are seen in subsequent-revision hip and knee arthroplasty for prosthetic joint infection, J. Bone Joint Infect., 8, 229–234, https://doi.org/10.5194/jbji-8-229-2023, 2023.
McNally, M., Sousa, R., Wouthuyzen-Bakker, M., Chen, A. F., Soriano, A., Vogely, H. C., Clauss, M., Higuera, C. A., and Trebse, R.: The EBJIS definition of periprosthetic joint infection: A practical guide for clinicians, Bone and Joint Journal, 103, 18–25, https://doi.org/10.1302/0301-620X.103B1.BJJ-2020-1381.R1, 2021.
Mitterer, J. A., Frank, B. J. H., Sebastian, S., Guger, M., Schoefberger, L., and Hofstaetter, J. G.: The Value of Preoperative Ultrasound-Determined Fluid Film and Joint Aspiration in Revision Hip Arthroplasty, J. Arthroplasty, 39, 1069–1074, https://doi.org/10.1016/j.arth.2023.10.029, 2024.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., and Wilson, W. R.: Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America, Clin. Infect. Dis., 56, e1–e25, https://doi.org/10.1093/cid/cis803, 2012.
Przekwas, J., Gębalski, J., Kwiecińska-Piróg, J., Wiktorczyk-Kapischke, N., Wałecka-Zacharska, E., Gospodarek-Komkowska, E., Rutkowska, D., and Skowron, K.: The effect of fluoroquinolones and antioxidans on biofilm formation by Proteus mirabilis strains, Ann. Clin. Microb. Anti., 21, 22, https://doi.org/10.1186/s12941-022-00515-5, 2022.
Rodríguez-Pardo, D., Pigrau, C., Lora-Tamayo, J., Soriano, A., del Toro, M. D., Cobo, J., Palomino, J., Euba, G., Riera, M., Sánchez-Somolinos, M., Benito, N., Fernández-Sampedro, M., Sorli, L., Guio, L., Iribarren, J. A., Baraia-Etxaburu, J. M., Ramos, A., Bahamonde, A., Flores-Sánchez, X., Corona, P. S., Ariza, J., Amat, C., Larrosa, M. N., Puig, M., Murillo, O., Cabo, X., Goenaga, M. Á., Elola, M., De la Herrán, G., Garcia-Arenzana, J. M., García-Ramiro, S., Martínez-Pastor, J. C., Tornero, E., García-Lechuz, J. M., Marín, M., Villanueva, M., López, I., Cisterna, R., Santamaría, J. M., Gómez, M. J., Puente, A., Cano, P., Horcajada, J. P., González-Mínguez, P., Portillo, E., Puig, L., Franco, M., Jordán, M., Coll, P., Amador-Mellado, J., Fuster-Foz, C., García-Paíno, L., Nieto, I., Muniain, M. Á., Suárez, A. I., Praena, J., Gómez, M. J., Puente, A., Maseguer, M. A., Garagorri, E., Pintado, V., Marinescu, C., Ramírez, A., Montaner, F., Múñez, E., Álvarez, T., García, R., Puente, E., Salas, C., Fariñas, M. C., Pérez, J. M., Achabal, B. V., and Montejo Baranda, J. M.: Gram-negative prosthetic joint infection: Outcome of a debridement, antibiotics and implant retention approach. A large multicentre study, Clin. Microbiol. Infec., 20, O911–O919, https://doi.org/10.1111/1469-0691.12649, 2014.
Schaffer, J. N. and Pearson, M. M.: Proteus mirabilis and Urinary Tract Infections, Microbiol. Spectr., 3, https://doi.org/10.1128/microbiolspec.uti-0017-2013, 2015.
Sebastian, S., Malhotra, R., Sreenivas, V., Kapil, A., Chaudhry, R., and Dhawan, B.: A Clinico-Microbiological Study of Prosthetic Joint Infections in an Indian Tertiary Care Hospital: Role of Universal 16S rRNA Gene Polymerase Chain Reaction and Sequencing in Diagnosis, Indian J. Orthop., 53, 646–654, https://doi.org/10.4103/ortho.IJOrtho_551_18, 2019.
Shohat, N., Bauer, T., Buttaro, M., Budhiparama, N., Cashman, J., Della Valle, C. J., Drago, L., Gehrke, T., Marcelino Gomes, L. S., Goswami, K., Hailer, N. P., Han, S. B., Higuera, C. A., Inaba, Y., Jenny, J. Y., Kjaersgaard-Andersen, P., Lee, M., Llinás, A., Malizos, K., Mont, M. A., Jones, R. M., Parvizi, J., Peel, T., Rivero-Boschert, S., Segreti, J., Soriano, A., Sousa, R., Spangehl, M., Tan, T. L., Tikhilov, R., Tuncay, I., Winkler, H., Witso, E., Wouthuyzen-Bakker, M., Young, S., Zhang, X., Zhou, Y., and Zimmerli, W.: Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? Can the Same Criteria be Used for Both Joints?, Proceedings of International Consensus on Orthopedic Infections, J. Arthroplasty, 34, S325–S327, https://doi.org/10.1016/j.arth.2018.09.045, 2019.
Tande, A. J. and Patel, R.: Prosthetic joint infection, Clin. Microbiol. Rev., 27, 302–345, https://doi.org/10.1128/CMR.00111-13, 2014.
Trampuz, A., Piper, K. E., Jacobson, M. J., Hanssen, A. D., Unni, K. K., Osmon, D. R., Mandrekar, J. N., Cockerill, F. R., Steckelberg, J. M., Greenleaf, J. F., and Patel, R.: Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection, New Engl. J. Med., 357, 654–663, https://doi.org/10.1056/nejmoa061588, 2007.
Uçkay, I. and Bernard, L.: Gram-negative versus gram-positive prosthetic joint infections, Clin. Infect. Dis., 50, 795, https://doi.org/10.1086/650540, 2010.
van Veghel, M. H. W., van Steenbergen, L. N., Wertheim, H. F. L., van der Kooi, T. I. I., Schreurs, B. W., and Hannink, G.: Early Periprosthetic Joint Infections in Total Hip and Knee Arthroplasty: Microorganisms, Mortality, and Implant Survival Using a Combined Dataset From the Dutch Arthroplasty Register and the Dutch National Nosocomial Surveillance Network, J. Arthroplasty, 40, 208–213, https://doi.org/10.1016/j.arth.2024.07.019, 2024.
Zhu, M. F., Kim, K., Cavadino, A., Coleman, B., Munro, J. T., and Young, S. W.: Success Rates of Debridement, Antibiotics, and Implant Retention in 230 Infected Total Knee Arthroplasties: Implications for Classification of Periprosthetic Joint Infection, J. Arthroplasty, 36, 305–310, https://doi.org/10.1016/j.arth.2020.07.081, 2021.
Zmistowski, B., Fedorka, C. J., Sheehan, E., Deirmengian, G., Austin, M. S., and Parvizi, J.: Prosthetic joint infection caused by gram-negative organisms, J. Arthroplasty, 26, 104–108, https://doi.org/10.1016/j.arth.2011.03.044, 2011.