the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
No differences in outcomes with stopping or continuing antibiotic suppression in periprosthetic joint infections
Megan Dunning
Sa Shen
Amy Chang
Jenny Aronson
Derek F. Amanatullah
Gina A. Suh
Shanthi Kappagoda
The data on long-term antibiotic use following debridement, antibiotics, and implant retention (DAIR) for treatment of periprosthetic joint infections are limited. In this single-center retrospective study, we show that patients with eventual cessation of antibiotic suppression after DAIR had similar outcomes to those who remained on chronic antibiotic suppression.
- Article
(645 KB) - Full-text XML
- BibTeX
- EndNote
Debridement, antibiotics, and implant retention (DAIR) is a surgical approach for treatment of periprosthetic joint infections (PJI) that is favored in certain patients such as those with short symptom duration, good soft tissue, and stable implants (Beam and Osmon, 2018; Osmon et al., 2013). The ideal antibiotic duration after DAIR is unclear. The 2012 Infectious Diseases Society of America (IDSA) PJI guideline states that indefinite oral antimicrobial suppression may follow standard treatment course and that the guideline panel could not agree on the use and duration of chronic antibiotic suppression (Osmon et al., 2013). The body of evidence is limited with a few retrospective studies suggesting potential benefit with antibiotic suppression (Byren et al., 2009; Bryan et al., 2017; Renz et al., 2019; Siqueira et al., 2015). However, as discussed in a review by Cortes-Penfield et al. (2024), these studies are highly heterogenous with regards to duration of suppression, definition of outcomes, and patient population, and it still remains unclear if and for whom antibiotic suppression is beneficial. In this study, we aimed to describe the failure rates in patients treated with DAIR and evaluate the role of long-term antibiotic suppression. We hypothesized that long-term antibiotic suppression would lower rates of failure.
We conducted a single-center retrospective study of patients who underwent DAIR for PJI. Patients with International Classification of Diseases 9th and 10th revision codes for PJI (996.6, T84.5, T84.6, T84.7) who were admitted and seen in our orthopedic-infectious diseases clinic from 1 January 2013 to 31 December 2019 were queried from our institution's clinical data warehouse. Patients with knee or hip PJIs who underwent DAIR were then identified through manual chart review. Exclusion criteria included PJI of other joints, tumor prosthesis, not meeting Musculoskeletal Infection Society (MSIS) criteria for PJI (Parvizi et al., 2018), and being lost to follow-up prior to completion of intravenous (IV) therapy. Patient characteristics, antibiotic type and duration, and laboratory values were abstracted through manual chart review and entered into REDCap. Treatment failure was defined as repeat surgery for clinical suspicion of infection leading to repeat treatment with a prolonged course of antibiotics recommended by the consulting infectious diseases provider. Antibiotic suppression was defined as oral antibiotics given after completion of IV therapy. Our standard practice at the time of this chart review was to treat patients with 6 weeks of IV therapy prior to transitioning to oral antibiotics. Antibiotics were selected based on IDSA guidelines, and rifampin was considered for patients with staphylococcal infections at the dose and duration recommended by the guideline (Osmon et al., 2013).
Our exploratory analysis identified that patients who did not meet guideline-recommended criteria for DAIR were included in our cohort. To account for this heterogeneity in patient cohort, we stratified our analysis based on whether the patient met criteria for DAIR as defined by the 2012 IDSA PJI guideline (well-fixed prosthesis without a sinus tract and within 30 d of prosthesis implantation or within 3 weeks of symptom onset) (Osmon et al., 2013).
Results were analyzed descriptively. We used the chi-square test and Fisher's exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables for group comparisons. We used STATA version 16 software (STATA Corp, College Station, TX) for all analyses.
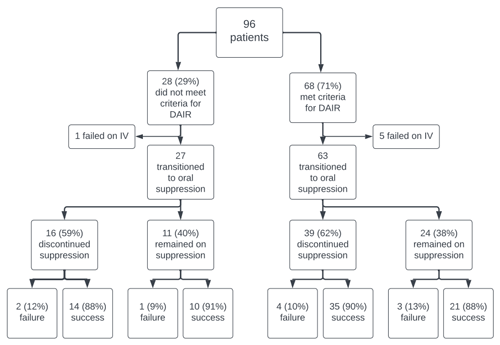
A total of 96 patients were included in our cohort of whom 68 (71 %) met criteria for DAIR. Of the 28 (29 %) patients who did not meet criteria for DAIR, the reason for not meeting criteria was prolonged symptom duration (n=25) and presence of sinus tract (n=3). The documented reasoning for pursuing DAIR despite not meeting criteria was that two-stage exchange was considered technically not feasible or challenging (n=9), infection was not suspected peri-operatively (n=5), patient preference (n=3), and unable to abstract from chart review (n=11).
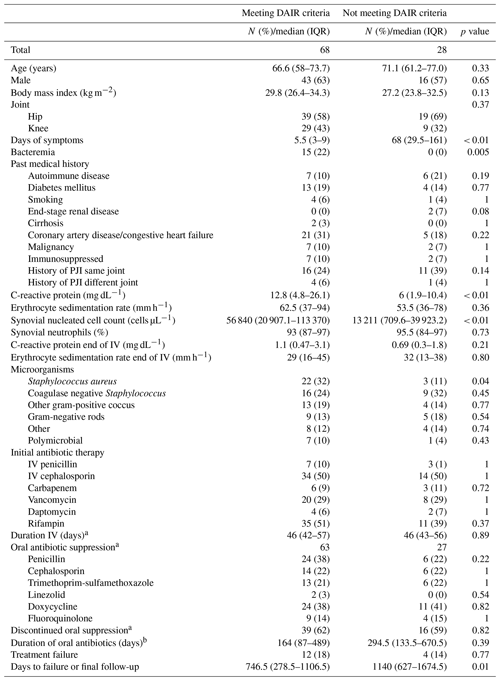
Table 1Baseline patient characteristics and comparison of patients grouped by treatment outcomes and status of oral suppression.
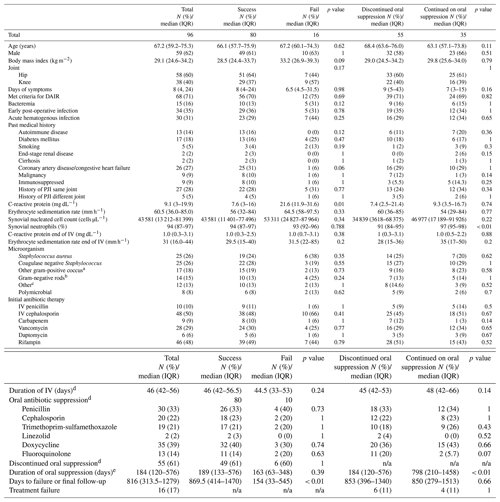
a Streptococcus spp. (n=13), Enterococcus spp. (n=4). b Enterobacterales (n=13), Pseudomonas aeruginosa (n=1). c Cutibacterium acnes (n=3), Corynebacterium spp. (n=2), Bacillus spp. (n=1), Burkholderia cenocepacia (n=1), Campylobacter fetus (n=1), Haemophilus parainfluenzae (n=1), Stenotrophomonas maltophilia (n=1), other anaerobes (n=3). d Analysis restricted to those that completed IV therapy (n=90). e Analysis restricted to those that discontinued suppression (n=55). n/a – not applicable
In the entire cohort, treatment failure was seen in 16 patients (17 %), of which 6 failed while on IV therapy and 12 failed within the first year. Median time to failure was 154 (IQR 33–545) days. Of 16 failures, 6 were due to the same organism as the index infection, and 4 were due to a different organism. The remaining 6 had negative cultures at time of repeat surgery, but all were on antibiotics when clinical failure was suspected. Rifampin was used in 46 (48 %) patients, with a median duration of 89 (IQR 58–145) days. Pre-operative C-reactive protein (CRP) levels were higher in the failure group compared to the success group (21.6 vs 7.6 mg dL−1, p=0.01), and there was no significant difference in the proportion of patients meeting criteria for DAIR (70 % success group vs 75 % failure group; p=0.69) or the proportion of patients remaining on suppressive antibiotic therapy (39 % success group vs 40 % failure group; p=1.0) (Table 1).
In the subset of patients who were transitioned to oral antibiotics, those who discontinued oral suppression had the same failure rates as those who were continued on oral suppression (11 % vs 11 %, p=1.0) (Table 1). Between the two groups, synovial percent neutrophil was the only factor that was significantly different (91 % [IQR 85–95] for the group that discontinued suppression vs 97 % [IQR 95–98] for the group that continued suppression; p<0.01), and there was no difference in the proportion of patients meeting criteria for DAIR (71 % vs 69 %; p=0.82) or median duration of follow-up (853 d vs 850 d; p=0.66). Incidence of treatment failure was similar regardless of the status of oral suppression, and this held true even after stratifying the analysis by meeting or not meeting DAIR criteria (Fig. 1). Patient data comparing those who did and did not meet criteria for DAIR are presented in Table A1 of the Appendix.
Overall, our study suggests that DAIR is an acceptable treatment strategy for patients with knee or hip PJIs, and our outcomes are even more reassuring as the majority of patients eventually stopped antibiotic therapy, sparing them from ongoing risk of adverse events Importantly, we demonstrated that outcomes were similar for patients who remained on suppressive antibiotics compared to those who discontinued suppression.
Other studies have demonstrated diminishing benefit with long term antibiotic use, arguing against indefinite suppressive antibiotics. Shah et al. (2020) showed that the benefit of suppression plateaued after 1 year of suppression, and Tai et al. (2022) similarly showed no differences in risk reduction between 1 year and 5 years of suppression. On closer observation of our data, three out of six patients who failed off suppression experienced failure either by a different organism or after over 2 years had passed since cessation of antibiotics. This may suggest that these failures were due to de novo acquisition of a new infection, rather than recurrence, thus overestimating rates of treatment failure. Though an argument could be made that being on suppressive antibiotics may have prevented new infections, the role of prophylactic antibiotics is not well established in this patient population and would not be a justification for indefinite suppression.
Interestingly, in our cohort, patients not meeting criteria for DAIR had similar overall failure rates to those who met criteria for DAIR, and the failure rates were similar even after stratifying the cohort by the status of chronic suppressive antibiotics. Though we would not suggest that DAIR should be considered for all patients regardless of timing of infection or symptom duration, our study does suggest that for some patients, outcomes may still be favorable even for those who do not meet guideline-recommended criteria for DAIR.
Our study has a number of limitations. First, this was a single-center retrospective study, and thus generalizability may be limited. Additionally, given the retrospective nature, there may have been bias unaccounted for in our analysis. Lastly, given the small sample size, our main findings were largely descriptive, and a more robust statistical analysis was not possible, which limited our ability to account for all confounders. We did however attempt to explore potential confounders by presenting data on patient characteristics based on DAIR criteria and antibiotic suppression. Notably, the major significant differences that we observed were that patients who met indication for DAIR had indices suggestive of acute inflammatory state (shorter symptom duration, higher CRP, higher synovial nucleated cells) (Table A1), which are expected differences considering the DAIR criteria. Regardless, based on our descriptive statistics alone, our study indicates that patients with eventual cessation of oral suppression still had high rates of success.
In our retrospective cohort study, patients with eventual cessation of antibiotics after DAIR had comparable outcomes to those who remained on indefinite oral antibiotic suppression.
Data used and analyzed are available upon reasonable request.
Conception: DF, SK, DFA, GAS. Data collection and abstraction: DF, JA, AC, MD. Study design and analysis: DF, SS. Manuscript preparation: DF with contributions from all authors).
At least one of the (co-)authors is a member of the editorial board of Journal of Bone and Joint Infection. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
The study was reviewed by our institution's Institutional Review Board.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Daisuke Furukawa was in part supported by the National Institutes of Health grant R25 AI 147369. Our institution's RedCap platform was in part supported by NIH grant UL1 TR001085.
This research has been supported by the Foundation for the National Institutes of Health (grant nos. R25 AI 147369 and UL1 TR001085).
This paper was edited by Rihard Trebse and reviewed by two anonymous referees.
Beam, E. and Osmon, D.: Prosthetic Joint Infection Update, Infect. Dis. Clin. North Am., 32, 843–859, https://doi.org/10.1016/j.idc.2018.06.005, 2018.
Bryan, A. J., Abdel, M. P., Sanders, T. L., Fitzgerald, S. F., Hanssen, A. D., and Berry, D. J.: Irrigation and Debridement with Component Retention for Acute Infection After Hip Arthroplasty: Improved Results with Contemporary Management, J. Bone Joint Surg. Am., 6, 2011–2018, https://doi.org/10.2106/JBJS.16.01103, 2017.
Byren, I., Bejon, P., Atkins, B. L., Angus, B., Masters, S., McLardy-Smith, P., Gundle, R., and Berendt, A.: One hundred and twelve infected arthroplasties treated with “DAIR” (debridement, antibiotics and implant retention): antibiotic duration and outcome, J. Antimicrob. Chemother., 63, 1264–1271, https://doi.org/10.1093/jac/dkp107, 2009.
Cortes-Penfield, N., Krsak, M., Damioli, L., Henry, M., Seidelman, J., Hewlett, A., and Certain, L.: How We Approach Suppressive Antibiotic Therapy Following Debridement, Antibiotics, and Implant Retention for Prosthetic Joint Infection, Clin. Infect. Dis., 25, 188–198, https://doi.org/10.1093/cid/ciad484, 2024.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., and Wilson, W. R.: Infectious Diseases Society of America, Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America, Clin. Infect. Dis., 56, e1–e25, https://doi.org/10.1093/cid/cis803, 2013.
Parvizi, J., Tan, T. L., Goswami, K., Higuera, C., Della Valle, C., Chen, A. F., and Shohat, N.: The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria, J. Arthroplasty, 33, 1309–1314, https://doi.org/10.1016/j.arth.2018.02.078, 2018.
Renz, N., Rakow, A., Müller, M., Perka, C., and Trampuz, A.: Long-term antimicrobial suppression prevents treatment failure of streptococcal periprosthetic joint infection, J Infect., 79, 236–244, https://doi.org/10.1016/j.jinf.2019.06.015, 2019.
Shah, N. B., Hersh, B. L., Kreger, A., Sayeed, A., Bullock, A. G., Rothenberger, S. D., Klatt, B., Hamlin, B., and Urish, K. L.: Benefits and Adverse Events Associated With Extended Antibiotic Use in Total Knee Arthroplasty Periprosthetic Joint Infection, Clin. Infect. Dis., 70, 559–565, https://doi.org/10.1093/cid/ciz261, 2020.
Siqueira, M. B., Saleh, A., Klika, A. K., O'Rourke, C., Schmitt, S., Higuera, C. A., and Barsoum, W. K.: Chronic Suppression of Periprosthetic Joint Infections with Oral Antibiotics Increases Infection-Free Survivorship, J. Bone Joint Surg. Am., 97, 1220–1232, https://doi.org/10.2106/JBJS.N.00999, 2015.
Tai, D. B. G., Berbari, E. F., Suh, G. A., Lahr, B. D., Abdel, M. P., and Tande, A. J.: Truth in DAIR: Duration of Therapy and the Use of Quinolone/Rifampin-Based Regimens After Debridement and Implant Retention for Periprosthetic Joint Infections, Open Forum Infect. Dis., 25, ofac363, https://doi.org/10.1093/ofid/ofac363, 2022.