the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Tuberculous arthritis of native joints – a systematic review and European Bone and Joint Infection Society workgroup report
Luan Nieuwoudt
Adisha Nansook
Aditya Menon
Natividad Benito
Introduction: The aim of this systematic review was to assess the existing published data on the diagnosis and management of tuberculosis (TB) arthritis involving native joints in adults aged 18 years and older. Methods: This study was performed in accordance with the guidelines provided in the Preferred Reporting Items for Systematic reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR). Results: The systematic review of the literature yielded 20 data sources involving 573 patients from nine countries. There was considerable variation amongst the studies in terms of the approach to diagnosis and management. The diagnosis was mostly made by microbiological tissue culture. Medical management involved a median of 12 months of anti-tubercular treatment (interquartile range, IQR, of 8–16; range of 4–18 months). The duration of preoperative treatment ranged from 2 to 12 weeks. Surgery was performed on 87 % of patients and varied from arthroscopic debridement to complete synovectomies combined with total joint arthroplasty. The mean follow-up time of all studies was 26 months (range of 3–112 months). Recurrence rates were reported in most studies, with an overall average recurrence rate of approximately 7.4 % (35 of 475 cases). Conclusions: The current literature on TB arthritis highlights the need for the establishment of standardized guidelines for the confirmation of the diagnosis. Further research is needed to define the optimal approach to medical and surgical treatment. The role of early debridement in active TB arthritis needs to be explored further. Specifically, comparative studies are required to address questions around the use of medical treatment alone vs. in combination with surgical intervention.
- Article
(673 KB) - Full-text XML
- BibTeX
- EndNote
Primary tuberculosis (TB) infection is usually acquired via the inhalation of Mycobacterium tuberculosis. During primary infection, hematogenous dissemination may occur and lead to the seeding of bacilli at many sites, including bones and joints, although it is generally contained by cell-mediated immunity. Manifestations of bone and joint TB are more common during the reactivation of latent bacilli, often many years after the initial infection, but may also occur during primary infection (Hogan et al., 2017). The latter is more common in young patients in highly endemic areas, whereas the former occurs mainly in adults (Pigrau-Serrallach and Rodríguez-Pardo, 2013; Johansen et al., 2015). The most frequent form of skeletal TB is spondylitis (Pott's disease), followed by arthritis (Johansen et al., 2015; Mateo et al., 2007; Qian et al., 2018).
The most common presentation of tuberculous arthritis is that of chronic granulomatous monoarthritis (Berney et al., 1972). Two varieties of tuberculous arthritis have been described: the first originates in the synovium and joint capsule, whereas the second primarily affects bone. The latter can be further divided into pre-arthritic, arthritic and post-arthritic (inactive or quiescent) stages (Titov et al., 2004). In a series from Italy, the mean time from the onset of symptoms to the initiation of treatment was 216 d, which reflects the indolent nature of the disease (non-specific symptoms) and the difficulty in making the diagnosis (Mariconda et al., 2007). Tuberculous arthritis can mimic pyogenic, fungal, non-tuberculous mycobacterial or inflammatory arthritis (Huang et al., 2007). The most commonly affected sites are the weight-bearing joints, namely the hip, knee, foot and ankle regions, followed by the shoulder, elbow and wrist (Gardam and Lim, 2005).
A recent systematic review looked at the disability resulting from tuberculosis and found that musculoskeletal impairment was the third most common, after mental heath disorders and respiratory impairment (Alene et al., 2021). Early initiation of treatment is considered key to preventing permanent impairment. While cultures remain the standard confirmatory approach, the paucibacterial nature of the disease leads to low culture yields (Agashe et al., 2020). This has led to the use of more rapid and sensitive methods, including nucleic acid amplification tests (NAATs) such as polymerase chain reaction (PCR) (Mohanty et al., 2022). Despite the advances in our diagnostic abilities, a high index of suspicion is still required. In non-endemic areas, a TB diagnosis is often not considered, and this may result in unnecessary delays (Broderick et al., 2018). Many questions remain regarding the optimal management of TB arthritis. Most data relate to spinal TB, and there is little to guide treatment of extra-axial involvement, particularly in terms of the duration of therapy (Hogan et al., 2017).
In 2019, the European Bone and Joint Infection Society (EBJIS) initiated an interdisciplinary collaborative project in order to create a concise evidence-based clinical guideline for the management of septic arthritis of native joints (SANJO). The steering committee identified specific clinical dilemmas related to SANJO and created workgroups of international experts to address these. The guidelines emanating from this project have recently been published, and the publication summarizes the recommendations of the workgroups (Ravn et al., 2023). In the present report, the workgroup on TB arthritis of native joints presents the findings of a systematic review that aimed to assess the existing published data on the diagnosis and management of TB arthritis in adults.
This study was performed in accordance with the guidelines provided in the Preferred Reporting Items for Systematic reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR) (Tricco et al., 2018).
2.1 Research questions
Specifically, we focused on the following questions:
-
When should the diagnosis of arthritis caused by Mycobacterium tuberculosis be suspected?
-
What tests should be performed in patients with suspicion of native joint infection caused by M. tuberculosis?
-
What is the recommended antimicrobial therapy for arthritis due to M. tuberculosis and its optimal duration?
-
What is the recommended surgical management of native joint infection caused by M. tuberculosis?
2.2 Eligibility criteria
All original peer-reviewed studies at all levels of evidence were considered eligible for inclusion, including randomized and non-randomized control trials; prospective and retrospective cohort studies; case control studies; and analytical, cross-sectional and observational studies. Only studies published in English since 1970 were considered. Case series involving less than 10 patients, systematic reviews, narrative reviews, and non-clinical (basic science) or animal studies were excluded. We also excluded reports involving paediatric patients under the age of 18 years as well as all TB infections not involving a “peripheral native joint”, like TB of the spine, pubis, sacroiliac joint, temporomandibular joint and sternoclavicular joint as well as TB tenosynovitis or dactylitis. Periprosthetic joint infection secondary to TB and rheumatoid arthritis with secondary TB were also excluded.
2.3 Information sources
A systematic literature search of PubMed, Web of Science, Scopus and the Cochrane Library was undertaken on 2 March 2023. MeSH terms used, as per the 2020 MeSH descriptor data, were as follows: “Joint” AND “Tuberculosis” AND “Humans” (Mesh) AND “English” (lang).
2.4 Search strategy
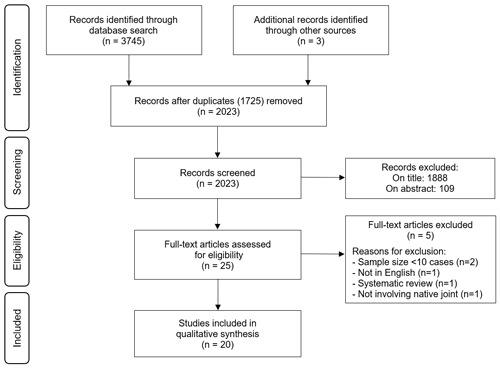
The phenomena of interest was defined as intervention aimed at the diagnosis and/or management of tuberculosis of native joints in adult patients 18 years or older. After performing the literature search, articles were first excluded based on the title and abstract (Fig. 1). All remaining articles were assessed based on the full text whilst considering the inclusion/exclusion criteria. The selection was performed independently by two reviewers (Leonard C. Marais and Luan Nieuwoudt), and disagreements were resolved by consensus.
2.5 Data extraction
Data descriptors that were extracted included authors, country of origin, year of publication, study design, number of participants and outcomes of interest. In terms of the outcomes of interest, the following data were extracted from each study: anatomic location of infection, diagnostic methods, medical and surgical management, and the outcomes and follow-up period.
2.6 Synthesis of results
Results were reported according to PRISMA-ScR (Tricco et al., 2018). Due to the paucity of data and the lack of high-level evidence, a qualitative description of the data was performed in the form of a thematic analysis in accordance with our research questions. The level of evidence and strength of recommendations was assessed in accordance with the GRADE (Grading of Recommendations, Assessment, Development and Evaluations).
The systematic review of the literature yielded 2023 potential sources, following deduplication, for screening. A description of the selection of the sources of evidence is depicted in Fig. 1. Following full-text review of 25 sources, 5 were excluded: 2 systematic reviews, 1 data source focused on total hip arthroplasty (not involving native joints), 1 publication in a language other than English and 2 series on extra-pulmonary TB that involved less than 10 cases of tuberculous arthritis. Thus, 20 data sources from nine countries were considered eligible for inclusion. The characteristics of the included studies are provided in Table 1.
Table 1Characteristics of included sources of evidence and clinical features.
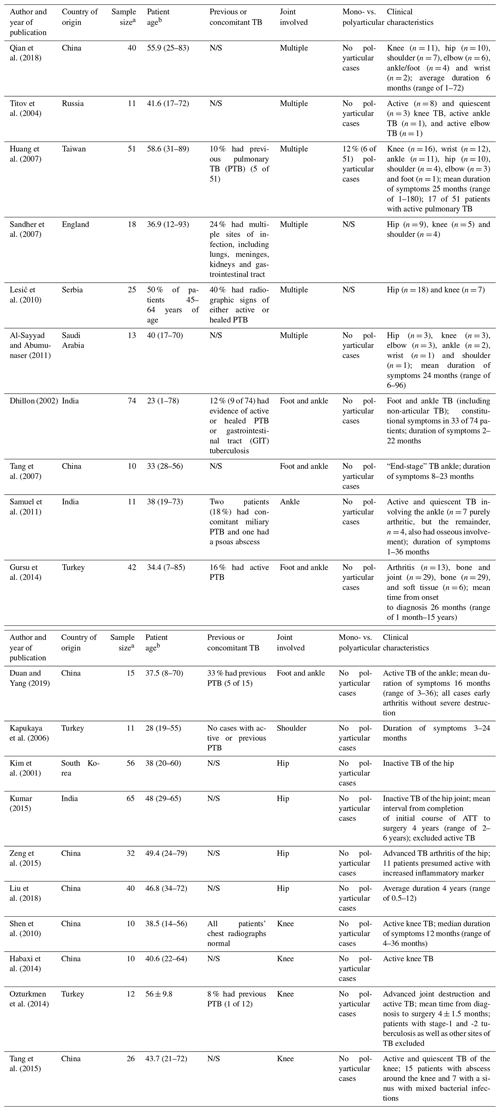
a Peripheral joints only, b Mean or median age in years (range), unless stated otherwise. The abbreviations used in the table are as follows: PTB – pulmonary tuberculosis; N/S – not stated; and GIT – gastrointestinal tract.
The included studies were highly heterogenous in terms of their inclusion criteria. In total, 573 patients with TB arthritis involving the extremities were included. In some studies, however, patients with a combination of bone and joint involvement were included, while other work did not clearly differentiate between those with pure joint or bone involvement. Most studies were observational and descriptive in nature, with one paper comparing the functional outcome following hip arthrodesis and arthroplasty (Liu et al., 2018).
3.1 Suspicion of TB arthritis
In the included studies, the clinical suspicion of TB arthritis was based on a combination of suggestive clinical and imaging findings. The preliminary clinical diagnosis was based on X-ray, computer tomography (CT) and/or magnetic resonance imaging (MRI) features; raised infection markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR); positive interferon-gamma release assays (IGRAs) or tuberculin skin tests; identification of acid-fast bacilli (AFB) on smear microscopy; and/or findings consistent with the diagnosis on histology (Table 2).
Table 2Diagnostic findings in terms of imaging and laboratory investigations as well as the confirmatory test used in the included studies.
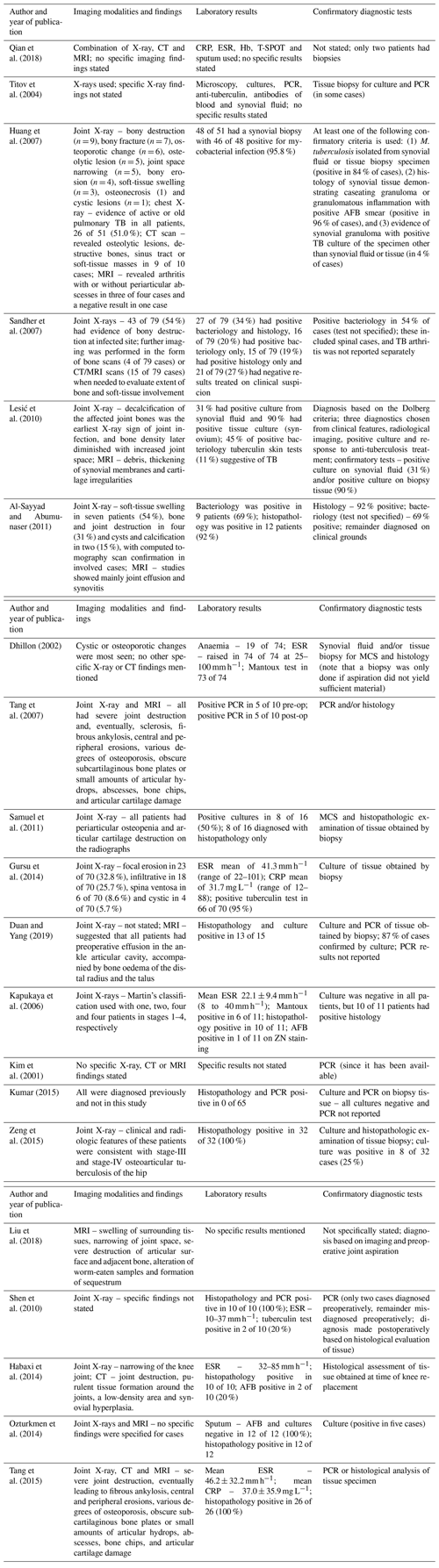
The abbreviations used in the table are as follows: Hb – haemoglobin; ZN – Ziehl–Neelsen; AFB – acid-fast bacilli; WBC – white blood cell count; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; PCR – polymerase chain reaction; ELISA – enzyme-linked immunosorbent assay; MCS – microscopy, culture and sensitivity testing; and N/S – Not stated.
Suggestive clinical symptoms and signs of TB arthritis included monoarthritis with an indolent course (weeks to months or even years), joint pain, swelling, effusion, a draining sinus, restricted movement and/or deformity (Al-Sayyad and Abumunaser, 2011; Qian et al., 2018; Sandher et al., 2007; Huang et al., 2007; Duan and Yang, 2019; Kapukaya et al., 2006; Habaxi et al., 2014). Suggestive general clinical features included low-grade fever, night sweats, weight loss, coexisting pulmonary symptoms, previous TB, or history of living or having previously lived in an endemic area (Al-Sayyad and Abumunaser, 2011; Qian et al., 2018; Sandher et al., 2007; Duan and Yang, 2019; Kapukaya et al., 2006; Habaxi et al., 2014). Typical X-ray findings were periarticular osteopenia, subchondral erosions, periarticular cysts or fractures, narrowing of the joint space, and/or bony destruction or sclerosis. Other imaging investigations included CT (Al-Sayyad and Abumunaser, 2011; Habaxi et al., 2014; Tang et al., 2015) and MRI (Al-Sayyad and Abumunaser, 2011; Lesić et al., 2010; Tang et al., 2007; Habaxi et al., 2014; Ozturkmen et al., 2014).
Some studies included IGRAs. Qian et al. (2018) used the T-SPOT ELISPOT assay, which was positive in 42.6 % of patients. Dhillon and Nagi (2002) reported that ELISA (enzyme-linked immunosorbent assay) was used as a diagnostic adjunct and found it to be positive in 12 of 17 patients with foot or ankle TB (Dhillon and Nagi, 2002). The specific target analyte of their test was not stated. Tang et al. (2015) reported the use of blood antibody testing but not the results of the tests.
The results of AFB smears were reported in a small number of series. Huang et al. (2007) reported that 63 % of smears were performed on joint fluid aspirates or tissue samples. Conversely, Gursu et al. (2014) and Kapukaya et al. (2006) reported that only 0 % and 10 % of tests were positive, respectively. The results of the histopathological assessment of tissue samples were reported in nine studies and found to be positive in 87 %–100 % of cases (Huang et al., 2007; Al-Sayyad and Abumunaser, 2011; Tang et al., 2007; Gursu et al., 2014; Duan and Yang, 2019; Kapukaya et al., 2006; Zeng et al., 2015; Ozturkmen et al., 2014). However, in some of these series, histology served as a confirmatory and inclusion criterion.
3.2 Confirmation of diagnosis
The most common method used to confirm the diagnosis of TB is microbiological culture of tissue obtained by biopsy (Table 2) (Huang et al., 2007; Sandher et al., 2007; Al-Sayyad and Abumunaser, 2011; Dhillon and Nagi, 2002; Tang et al., 2007; Samuel et al., 2011; Gursu et al., 2014; Duan and Yang, 2019; Kapukaya et al., 2006; Kim et al., 2001; Kumar et al., 2015; Zeng et al., 2015; Shen et al., 2010; Habaxi et al., 2014; Ozturkmen et al., 2014; Tang et al., 2015; Liu et al., 2018). Overall, positive culture results ranged from 25 % to 100 % of cases. In some series, M. tuberculosis microbiological culture was also performed on synovial fluid aspirates (Huang et al., 2007; Dhillon and Nagi, 2002; Liu et al., 2018). Lešić et al. (2010) observed positive cultures in 31 % of joint fluid samples, whereas 90 % of tissue samples produced positive cultures. Huang et al. (2007) found positive histopathology in 96 % of synovial biopsies, while M. tuberculosis was cultured in 84 % of synovial tissue and/or fluid samples. AFB smear of synovial fluid or tissue was positive in only 63 % of cases. Al-Sayyad and Abumunaser (2011) reported positive histology in 92 % of cases and positive microbiology in 69 % of cases; however, neither the nature of the samples nor the method of analysis was noted. Zeng et al. (2015) reported positive histopathology in 100 % of their 32 patients, and positive M. tuberculosis culture on joint fluid and tissue was noted in 25 % of cases. In the studies that reported explicitly on the culture results (excluding studies including spine cases), the combined positive rate was 93 % (126 of 136 cases).
In some series, the diagnosis was confirmed through NAATs. PCR was used in several studies (Titov et al., 2004; Tang et al., 2007; Kim et al., 2001; Kumar et al., 2015; Duan and Yang, 2019). Kumar et al. (2015) reported that they performed PCR analysis on fluid and tissue obtained during surgery in all patients. The diagnostic accuracy of the test was not reported. The other studies did not report on the number of cases that were PCR-positive in comparison to culture.
In some series, the diagnostic criteria and methodology were not clearly defined and a combination of criteria were used, including clinical suspicion based on clinical history and physical examination, raised infection markers, Mantoux test results, radiological appearance (on X-rays, CT and/or MRI), response to anti-tuberculous therapy (ATT), microbiological analysis and/or histopathology (Qian et al., 2018; Titov et al., 2004; Sandher et al., 2007; Lesić et al., 2010). Lešić et al. (2010) established the diagnosis of TB via the application of the criteria put forth by Dolberg et al. (1991). According to this, the diagnosis of TB is made if any three of the following four criteria are met: typical clinical manifestation, radiological picture, positive M. tuberculosis culture or positive response to ATT.
3.3 Medical treatment
There was significant diversity in the approach to medical management demonstrated among the studies included in the data synthesis (Table 3). Most stated that they were following their respective national tuberculosis treatment guidelines. Overall, the medical management involved a median of 12 months of ATT (interquartile range, IQR, of 8–16; range of 4–18 months). In the studies that specified preoperative ATT, the median duration of preoperative ATT was 3 weeks (IQR of 2–4; range of 1–4 weeks) and the postoperative median duration was 12 months (IQR of 6–16; range of 2–18 months). There was considerable variation in the ATT agents used amongst the included studies. In collating these results, five groups emerged according to the anatomical site of infection, consisting of hip, knee, foot and ankle, shoulder, and mixed cohorts.
Table 3Management strategies employed and outcomes reported in the included sources of evidence.
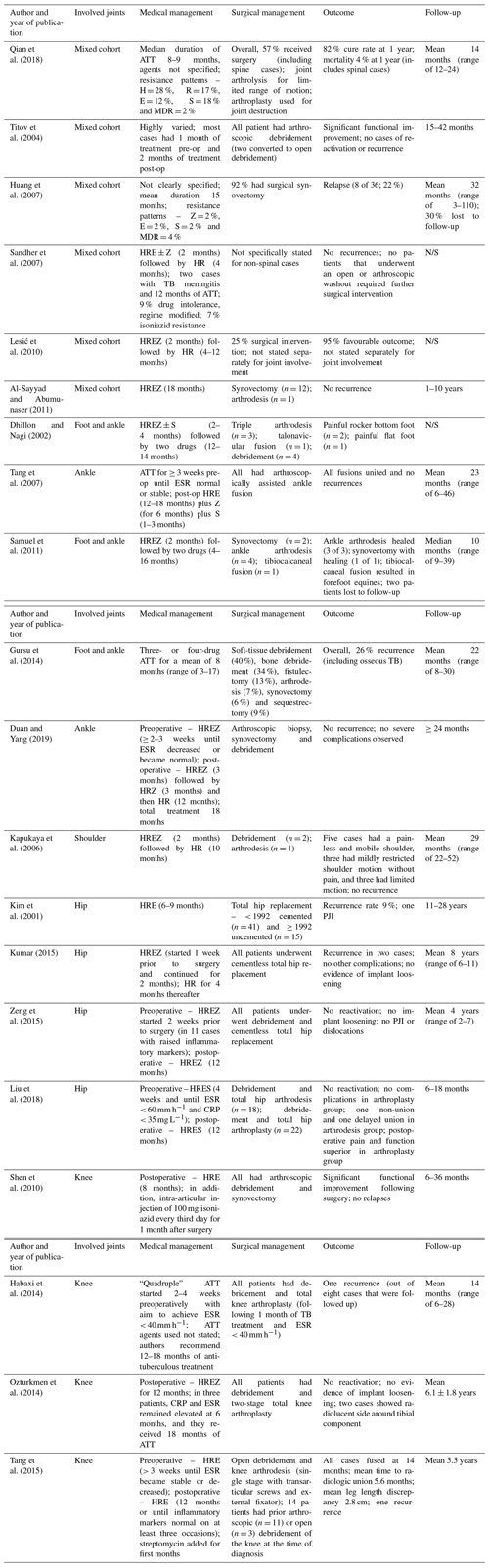
The abbreviations used in the table are as follows: H – isoniazid; R – rifampin; E – ethambutol; Z – pyrazinamide; S – streptomycin; MDR – multidrug resistant; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; PJI – periprosthetic joint infection; and N/S – not stated.
The four studies on TB of the hip reported preoperative medical treatment consisting of a four-drug combination containing isoniazid (H), rifampicin (R), ethambutol (E), and pyrazinamide (Z) or streptomycin (S) for 1–4 weeks. Postoperatively, this regime was continued for between 6 and 12 months, most favouring not to de-escalate to two-drug regimens (three out of four series). Kim et al. (2011) reported on 56 hips with an 11- to 28-year follow-up period (Kim et al., 2001). Their typical preoperative treatment period was 3 months, and the postoperative treatment comprised 6 months with a three-drug (HRE) combination. Kumar et al. (2015) had a 1-week preoperative and a 2-month postoperative HREZ regimen, de-escalating to HR for a further 4 months. Zeng et al. (2015) and Liu et al. (2018) followed a similar 2 or 4 week preoperative regimen of HREZ or HRES and both continued for 12 months postoperatively using the same drugs.
Four studies focused on TB of the knee joint (Shen et al., 2010; Ozturkmen et al., 2014; Tang et al., 2015; Habaxi et al., 2014). Medical treatment followed a similar trend with preoperative three-drug (HRZ) or four-drug (HREZ) regimes for a period of 2–4 weeks, followed by the same drugs postoperatively for 8–18 months. Shen et al. (2010) reported 10 cases that all underwent arthroscopic debridement and an 8-month postoperative HRE drug administration. Ozturkmen et al. (2104) employed only postoperative ATT involving a 12-month HREZ protocol, while Tang et al. (2015) used HRE±S for 3 weeks pre-op and 12 months post-op. Habaxi et al. (2014) was unclear on the exact anti-tubercular agents that were used, but they recommended 12–18 months of treatment in total. In their series, ATT was given for 2–4 weeks prior to arthroplasty, aiming for an ESR < 40 mm h−1.
In studies involving TB of the foot and/or ankle, a lower proportion of patients received surgery (Dhillon and Nagi, 2002; Tang et al., 2007; Samuel et al., 2011; Gursu et al., 2014). Preoperative treatment periods were between 8 and 12 weeks, consisting of four-drug combinations, in four out of five studies. This was ensued by two- to four-drug combinations for between 4 and 18 months post-op (median 11 months). In Gursu et al. (2014), the mean treatment duration was 8 months (range of 3–17 months) utilizing a three- or four-drug regimen.
One study (Kupukaya et al., 2006) investigating TB of the shoulder also used a four-drug (HREZ) regime for 8 weeks before surgery, switching to a two-drug (HR) 10-month strategy after debridement or fusion surgery.
Medical treatment extrapolation from the mixed-cohort studies was more difficult, with most studies not clearly specifying their exact protocols (Qian et al., 2018; Titov et al., 2004; Huang et al., 2007; Al-Sayyad and Abumunaser, 2011). The studies that did state their chemotherapy regimens favoured 2 months of three or four drugs (HRE or HREZ, respectively) pre-op and a two-drug (HR) regime post-op (Sandher et al., 2007; Lesić et al., 2010).
3.4 Surgical treatment
Surgery was performed on at least some of the patients in all of the included studies. For 81 patients, the only surgical procedure was a biopsy for diagnostic purposes (Qian et al., 2018; Sandher et al., 2007; Gursu et al., 2014; Kapukaya et al., 2006). Curative surgical interventions varied, ranging from debridement to total joint arthroplasty. The overall surgical rate was 82 % (367 of 477 patients) in those studies that reported this information explicitly. When arthroplasty and arthrodesis are excluded, 86 of 107 patients (80 %) received an open or arthroscopic debridement (Huang et al., 2007; Titov et al., 2004; Al-Sayyad and Abumunaser, 2011; Samuel et al., 2011; Duan and Yang, 2019; Kapukaya et al., 2006).
The studies involving TB of the hip joint had a total of 193 patients with a 100 % surgical intervention rate, 91 % being total hip arthroplasty (THA) and the remaining 9 % being hip arthrodesis (Kumar et al., 2015; Kim et al., 2001; Zeng et al., 2015; Liu et al., 2018). Uncemented implants were the most common prostheses used in 77 % (134 of 175) of cases. Kumar et al. (2015), Zeng et al. (2016) and Liu et al. (2018) collectively reported no osteolysis, stem subsidence nor cup loosening. Kumar et al. (2015) reported on 65 cases of TB hip. All patients completed 18 months of ATT and had proven resolution of active disease, both radiologically and biochemically, before they underwent uncemented total hip arthroplasty. No early or late surgical complications were recorded, but they did have two recurrences not requiring surgery. Zeng et al. (2015) also described no surgical complications in their cohort of 32 uncemented THA cases. Liu et al. (2018), who compared arthroplasty with arthrodesis in TB hip, echoed this finding in their arthroplasty group of 22 patients.
Similarly, all 58 patients with TB knee had surgery (Shen et al., 2010; Habaxi et al., 2014; Ozturkmen et al., 2014; Tang et al., 2015). Shen et al. (2010) reported 10 cases of early TB knee that all underwent arthroscopic debridement. No sinuses nor wound complications occurred, with all patients reported as having good outcomes and an increased range of knee motion. Habaxi et al. (2014) performed total knee arthroplasty (TKA) on 10 active cases of TB knee. Four patients had open sinuses at the time of recruitment, but all had closed sinuses at the time of surgery. No aseptic loosening, fractures nor dislocations occurred postoperatively. Ozturkmen et al. (2014) described 12 cases of TKAs in advanced TB knee; equivalently, they reported no pain, loosening nor any other surgical complications. The last study (Tang et al., 2015) looked at end-stage disease exclusively. All 26 patients underwent knee arthrodesis with cross-screws and a unilateral external fixator. Most (96 %) had fused successfully at 8 months post-op, and all had fused successfully at 14 months post-op. Complications included one patient who developed a sinus far from the site of surgery (greater trochanter area) that resolved with minor surgery and medical treatment alone.
In the studies looking at TB of the foot and/or ankle, the overall surgical intervention rate was 35 % (excluding biopsies) (Dhillon and Nagi, 2002; Tang et al., 2007; Samuel et al., 2011; Gursu et al., 2014; Duan and Yang, 2019). Surgical debridement was the most favoured procedure in 79 % of cases, and it was performed either open or arthroscopically. Dhillon and Nagi (2022) noted that only 5 out of 74 (7 %) of their patients required surgery: 3 had triple arthrodeses, 1 had a talonavicular fusion and 1 had two synovectomies. Two patients developed painful rocker bottom feet, and one patient had a painful spastic flat foot. Gursu et al. (2014) also performed a similar descriptive study. Here, 28 of 70 patients required additional procedures: 40 % underwent soft-tissue debridement, 34 % underwent bone debridement and 7 % underwent arthrodesis. At final follow-up, 56 % of patients had either severe bony destruction or end-stage arthrosis. In the cohort of Samuel et al. (2011), 7 out of the 16 (44 %) patients had surgery: 5 needed ankle fusion procedures and 2 required ankle synovectomies. Tang et al. (2007) looked at end-stage ankle TB cases that had arthroscopic joint preparation. All had fused at 14.5 weeks post-op using a ring fixator compression technique following joint preparation, and no complications were reported. Lastly, Duan et al. (2019) observed no adverse events in 15 cases of early-stage disease treated with arthroscopic debridement.
Kupukaya et al. (2006) analysed shoulder tuberculosis in 11 patients with a 27 % (3 of 11) surgical intervention rate. This comprised debridement in two cases and arthrodesis in the other. Pain-free shoulders were found at final follow-up.
A total of 6 of our 22 included studies comprised a mixture of involved joints (Qian et al., 2018; Titov et al., 2004; Huang et al., 2007; Al-Sayyad and Abumunaser, 2011; Sandher et al., 2007; Lesić et al., 2010). The mixed-cohort studies were largely comprised of spinal tuberculosis (39 %), followed by hip (14 %) and knee tuberculosis (11 %). These six studies had an overall surgical intervention rate of 44 % with an expected high heterogeneity due to the nature of this mixed cohort (Table 1).
Table 4Consensus recommendations formulated by the EBJIS workgroup on tuberculous arthritis.
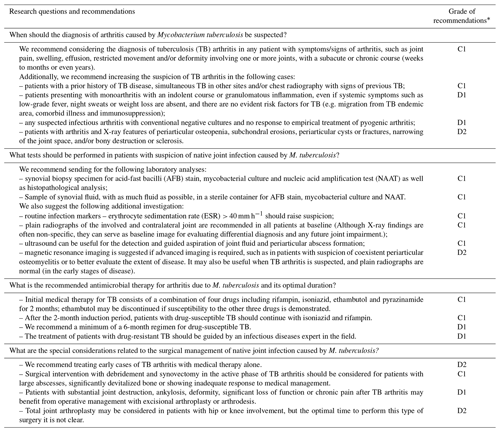
* The GRADE (Grading of Recommendations, Assessment, Development and Evaluations) quality of evidence is presented as follows: A – high, B – moderate, C – low, and D – very low. The associated strength of recommendation is presented as follows: 1 – strong recommendation for or against an intervention and 2 – weak recommendation for or against an intervention.
3.5 Outcomes
The combined mean follow-up time of all studies was 26 months, with most demonstrating a minimum follow-up time of at least 6 months (range of 3–112 months). Recurrence rates were reported in most studies, with an overall average recurrence rate of 7.4 % (35 of 475). The average recurrence rate was 11.9 % in those studies that did observe cases of recurrence (35 of 252) (Kim et al., 2001; Kumar et al., 2015; Habaxi et al., 2014; Tang et al., 2015). A total of 10 studies reported no recurrences (0 of 223 patients) (Kapukaya et al., 2006; Zeng et al., 2015; Shen et al., 2010; Ozturkmen et al., 2014). It is not clear if there is any association between the duration of ATT and the rate of recurrence.
Only one study highlighted specific factors related to recurrence (Huang et al., 2007). The presence of multi-focal arthritis, drug-resistant M. tuberculosis strains and poor compliance with treatment were deemed to be the lead causative factors. The timeframe of disease recurrence was noted in two studies. The mean time to recurrence was 6 months, ranging from 3 to 9 months following completion of treatment (Huang et al., 2007; Habaxi et al., 2014). These recurrences were recognized by a combination of clinical features (presence of a new sinus or ongoing pain and swelling) and elevated inflammatory markers (CRP and ESR). The combined recurrence rate in the TB hip group was 4 %. Kim et al. (2001) stated that their 9 % recurrence rate was mostly due to improper chemotherapy administration due to interdepartmental communication errors or primary drug resistance. Kumar et al. (2015) encountered a 3 % recurrence with a 6-month treatment duration (2 months of HREZ followed by 4 months of HR), whereas Zeng et al. (2015) and Liu et al. (2018) observed no reactivation following 12 months of postoperative treatment consisting of four agents (HREZ/HRES). The recurrence rate in TB of the knee ranged from 0 to 4 % (Shen et al., 2010; Habaxi et al., 2014; Ozturkmen et al., 2014; Tang et al., 2015). The highest recurrence rate of 26 % was reported in the foot and ankle group (Gursu et al., 2014). Neither Tang et al. (2007) nor Duan et al. (2019) reported recurrences, while the other studies in the foot and ankle group did not specifically report on their recurrence rates. In terms of recurrence rates in the mixed-cohort group, Titov et al. (2004) and Sandher et al. (2007) found no recurrences. Qian et al. (2018) reported an overall cure rate of 82 %. Lešić et al. (2010) reported favourable outcomes in 94 % of cases. These two studies were on mixed cohorts, and the outcome measures were not explicitly defined. Dhillon and Nagi (2002) proposed the following criteria for a successful outcome and healing in foot and ankle TB: (1) resolution of local symptoms such as pain, swelling and healing of the sinuses; (2) decrease in the serial ESRs; and (3) radiologic evidence showing remineralization, decrease in osteoporosis and fusion in patients in whom arthrodesis was done.
Some studies also reported the functional outcomes or the outcomes of surgery. Titov et al. (2004), for example, reported significant functional improvement after arthroscopic debridement of the knee in terms of the range of motion (ROM) and Knee Scoring System. Similarly, Shen et al. (2010) used the ROM and the Japanese Orthopaedic Association knee rating system. Kapukaya et al. (2006) reported outcomes in terms of residual pain and ROM following treatment. Tang et al. (2007, 2015) and Samuel et al. (2011) reported a 100 % fusion rate of ankle and knee arthrodesis, while Liu et al. (2018) observed one non-union and one delayed union out of their 18 hip arthrodesis cases. Two series reported a 0 % implant loosening of hip and knee arthroplasty at a mean follow-up of 8 and 6 years, respectively (Kumar et al., 2015; Ozturkmen et al., 2014). We could not ascertain if surgical debridement plus ATT had favourable outcomes compared with ATT alone. The lack of data, variation with respect to methodology and lack of specific comparative studies focusing on this precluded it.
The aim of this systematic review was to assess the available evidence on the diagnosis and management of tuberculous arthritis of native joints. The data emanating from this systematic review and the opinion of experts were then used to inform the international workgroup's recommendations. With regards to the specific research questions, the workgroup's consensus recommendations are provided in Table 4.
One of the main challenges in joint TB is the diagnosis, particularly because of its low incidence (especially in non-endemic areas), the usual absence of simultaneous lung disease and constitutional TB symptoms, and its indolent course with scarce inflammatory signs. TB arthritis should be considered in any patient presenting with a chronic granulomatous monoarthritis of insidious onset (Hogan et al., 2017; Berney et al., 1972). Previous history of tuberculosis (present in < 25 % of cases) and chest X-ray changes suggestive of active or old pulmonary tuberculosis (present in < 50 % of cases) should increase the suspicion of TB arthritis, even though these features are not ubiquitous (Mariconda et al., 2007; Huang et al., 2007). An ESR > 40 mm h−1 should also raise suspicion (Mariconda et al., 2007; Huang et al., 2007; Dhillon and Nagi, 2002; Zeng et al., 2015). Imaging modalities, including routine radiography, ultrasound, CT and MRI, are useful adjuncts to the clinical work-up (Venkat et al., 2018). With these clinical findings being relatively non-specific, it is important to confirm the clinical suspicion of TB with an accurate and reliable diagnostic test. Our results suggest considerable variation in the way the diagnosis was made in the literature, and there is a need for standardization of the diagnostic criteria. AFB smears of synovial fluid have a low yield and are of little diagnostic value (Berney et al., 1972). Similarly, joint fluid cultures may only be positive in 25 % of cases (Allali et al., 2005). In most of the series in this systematic review, the diagnosis was made by histological analysis and/or microbiological culture from tissue obtained through biopsy. Histological evaluation and AFB smears are, however, currently not considered to be stand-alone definitive confirmatory tests, as they may be positive in non-tuberculous mycobacterial infections. A recent meta-analysis looking at the value of IGRAs performed on whole blood found a pooled sensitivity of 84 % and a specificity of 78 %. The authors concluded that IGRA on whole blood exhibits poor diagnostic accuracy in osteoarticular TB (Ren et al., 2022). The gold standard for diagnosis is currently considered to be the identification or isolation of M. tuberculosis by culture or NAAT, such as PCR (Hogan et al., 2017; Lewinsohn et al., 2017). Mycobacterial susceptibility study should always be performed on culture isolates. The diagnosis of TB arthritis often requires the microbiological study of synovial tissue, which is more sensitive (90 % of positive cultures) than synovial fluid analysis alone (Hogan et al., 2017; Bennet et al., 2020). Additional sampling of periarticular fluid collections may also be of value. Microbiological culture has traditionally been considered the most sensitive test, but its main drawback is the prolonged time (generally several weeks) it takes and low yield rates (Agashe et al., 2020). Alternative culture methods, like the BACTEC Myco/F lytic and MGIT960 systems, appear to be superior to Löwenstein–Jensen medium for the culture of body fluid and tissue samples (Wang et al., 2016; Zhao et al., 2023). However, limited data are available on the use of these media for articular TB specifically. NAATs can provide a rapid diagnosis; the Xpert MTB/RIF assay is an automated NAAT that can simultaneously detect M. tuberculosis and rifampin resistance, and it has also demonstrated good diagnostic accuracy for bone and joint TB (Shen et al., 2010; Li et al., 2018; Gu et al., 2015). This test, however, is not currently approved by the FDA (Food and Drug Administration) for samples other than sputum in the United States. Furthermore, the processing of osteoarticular specimens for PCR is important, and a standardized approach would be beneficial when comparing findings. Current data suggest that the more recently developed Xpert Ultra may increase the yield (Sun et al., 2019). Although a positive NAAT confirms the diagnosis, a negative result cannot rule out TB. Few data are available on the sensitivity and specificity of NAAT in the setting of culture-negative TB arthritis (Hogan et al., 2017).
Antimicrobial therapy remains the cornerstone of treatment. While some cases may be managed with medical therapy alone, surgical intervention may also be warranted (Hogan et al., 2017; Pigrau-Serrallach and Rodríguez-Pardo, 2013; Shen et al., 2010; Tuli, 2002). In early cases, ATT can result in complete resolution, without residual joint disease (Pigrau-Serrallach and Rodríguez-Pardo, 2013). This supports the importance of a high level of suspicion of joint TB and an early, aggressive approach to diagnosis. According to the Centers for Diseases Control and Prevention (CDC) guidelines, a 6- to 9-month regimen containing rifampin is recommended for the treatment of bone and joint TB (Nahid et al., 2016). Initial medical therapy for drug-susceptible TB consists of a combination of four drugs, including rifampin, isoniazid, ethambutol and pyrazinamide, for 2 months (ethambutol may be discontinued if susceptibility to the other three drugs is demonstrated) (Hogan et al., 2017; Nahid et al., 2016). After this induction period, patients with drug-susceptible TB should continue with isoniazid and rifampin for a minimum of 4 to 7 more months of therapy (Nahid et al., 2016). However, the optimal duration of ATT for bone and joint TB remains uncertain (Shen et al., 2010). Most of the information comes from older studies on spinal TB, with various limitations (Anonymous, 1986, 1993; Darbyshire, 1999). Our systematic review revealed considerable variation in the approach to medical management, with the duration of preoperative treatment varying from 2 to 12 weeks and postoperative treatment consisting of two to four drugs for anything from 4 to 18 months. Some experts tend to favour 9- to 12-month duration regimens, particularly in patients who present with a significant burden of disease or a high net state of immunosuppression (Shen et al., 2010). ATT should ideally be supervised by an expert in the field, particularly in the case of multidrug-resistant TB (MDR-TB). In the case of MDR-TB, the Infectious Diseases Society of North America (IDSA) recommends an intensive phase duration of 5–7 months (five drugs) and a total duration of 15–20 months following culture conversion (with four drugs for the continuation phase) (Nahid et al., 2019).
While the backbone of treatment of joint TB remains ATT, surgery is necessary in some cases, although its role in not always clear (Tuli, 2002). Surgical intervention in the active phase of TB arthritis is usually considered for patients with large abscesses, significant devitalized bone and/or those showing an inadequate response to medical management (Tuli, 2002; Lesić et al., 2010; Gursu et al., 2014). However, some authors have suggested that surgical debridement and synovectomy may expedite healing and limit joint damage (Vohra et al., 1997; Dhillon and Nagi, 2002; Samuel et al., 2011; Duan and Yang, 2019; Gardam and Lim, 2005). There is, however, little evidence in the literature to support this premise, as clinical trials or even sound observational studies with control groups are lacking. Further studies would be needed to determine if the addition of arthroscopic or open debridement is superior to medical management alone. Although total joint arthroplasty is typically not performed during the active phase of TB arthritis, it may be unavoidable in certain instances. In these cases, arthroplasty followed by long-term ATT may be used with some success (Hogan et al., 2017). While active infection was previously considered to be a contraindication for arthroplasty surgery, with long intervals of at least 10 years being advocated, there have been a number of reports of total hip arthroplasty (THA) in cases with active TB infection (Tuli, 2002; Duan and Yang, 2019). A previous systematic review on the outcome of single-stage THA in 65 patients with active hip TB found only one case of reactivation in a patient who was non-compliant with treatment after a mean follow-up of 53 months (2–9 years) (Kim et al., 2013). The evidence for TKA in patients with active knee TB, however, remains limited (Habaxi et al., 2014). Most authors emphasize the importance of a thorough debridement when doing arthroplasty in the setting of active infection. Zeng et al. (2016) performed a two-stage TKA following 3 months of preoperative ATT in four cases with active infection. At a mean follow-up of 4 years (range of 2–7 years), there was no reactivation of infections. Patients with quiescent infection and substantial joint destruction, fibrous ankylosis with significant loss of function and/or chronic pain after TB arthritis may also benefit from operative management (Hogan et al., 2017; Tuli, 2002). Excisional arthroplasty, joint replacement surgery or arthrodesis may be considered to treat these sequalae of TB arthritis (Hogan et al., 2017; Dhillon and Nagi, 2002; Tuli, 2002). For the hip and knee, joint replacement appears to be superior in terms of the functional outcome (Liu et al., 2018; Kumar et al., 2015; Zeng et al., 2015, 2016). Optimally, total joint arthroplasty after TB arthritis should be deferred until patients show no evidence of recurrent disease after completion of therapy, given the potential for reactivation disease after arthroplasty (Hogan et al., 2017). However, the optimal time interval between the treatment of joint TB and the arthroplasty surgery is not known. In most cases, patients undergoing joint replacement received prolonged pre- and postoperative ATT.
There are a number of limitations to this study. Firstly, the search terms were specifically chosen to yield as many data sources as possible. Despite this, articles focusing on certain specific aspects of joint TB may not have been included. For example, studies comparing the accuracy of the various diagnostic modalities of TB were not included. Secondly, with the focus being tuberculosis of the native joint, we may have missed data from studies looking at bone TB that included cases with joint involvement. We excluded case reports and small case series with less than 10 patients as well as papers from before 1970. In studies looking at a mixed cohort of cases, the diagnosis, management and outcomes were often not specifically reported per joint site, making any quantitative analysis problematic. There were few comparative studies; therefore, it was not possible to make definitive recommendations supported by high-level evidence. The lack of comparative studies focusing on the outcomes of surgical and non-surgical treatment hampered our ability to explore this subject any further. There is a dearth of data providing insight into the factors associated with an increased risk of the recurrence of infection. In the same vein, it remains unclear if the duration of ATT has a role to play in decreasing recurrence rates. If anything, this study has highlighted the great need for well-designed prospective cohort studies or randomized trials to shed more light on the many questions that remain.
The current literature on TB arthritis highlights the need for the establishment of standardized diagnostic criteria. Further research is needed to define the optimal approach to medical and surgical treatment and the timing and technique of reconstruction procedures. The role of early debridement in active TB arthritis needs to be explored further. Specifically, comparative studies are required to address the questions around the use of medical treatment alone vs. in combination with surgical intervention.
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
LCM was part of the workgroup that developed proposals, performed the systematic review, extracted data, and prepared and revised the manuscript. LN performed the systematic review and prepared and reviewed the manuscript. AN extracted data and prepared and reviewed the manuscript. AM and NH were part of the workgroup that developed the proposals, assisted with literature review, and prepared and reviewed the manuscript.
The contact author has declared that none of the authors has any competing interests.
Ethics committee approval was not required for this systematic review.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors would like to thank Christen Ravn for the leadership he provided in order to take this project to completion.
This paper was edited by Bryan Springer and reviewed by two anonymous referees.
Anonymous: A controlled trial of six-month and nine-month regimens of chemotherapy in patients undergoing radical surgery for tuberculosis of the spine in Hong Kong, Tenth report of the Medical Research Council Working Party on Tuberculosis of the Spine, Tubercle, 67, 243–259, https://doi.org/10.1016/0041-3879(86)90014-0, 1986.
Anonymous: Controlled trial of short-course regimens of chemotherapy in the ambulatory treatment of spinal tuberculosis. Results at three years of a study in Korea. Twelfth report of the Medical Research Council Working Party on Tuberculosis of the Spine, J. Bone Joint Surg. Br., 75, 240–248, https://doi.org/10.1302/0301-620x.75b2.8444944, 1993.
Agashe, V. M., Johari, A. N., Shah, M., Anjum, R., Romano, C., Drago, L., Sharma, H. K., and Benzakour, T.: Diagnosis of Osteoarticular Tuberculosis: Perceptions, Protocols, Practices, and Priorities in the Endemic and Non-Endemic Areas of the World-A WAIOT View, Microorganisms, 8, https://doi.org/10.3390/microorganisms8091312, 2020.
Alene, K. A., Wangdi, K., Colquhoun, S., Chani, K., Islam, T., Rahevar, K., Morishita, F., Byrne, A., Clark, J., and Viney, K.: Tuberculosis related disability: a systematic review and meta-analysis, BMC Med., 19, 203, https://doi.org/10.1186/s12916-021-02063-9, 2021.
Allali, F., Mahfoud-Filali, S., and Hajjaj-Hassouni, N.: Lymphocytic joint fluid in tuberculous arthritis. A review of 30 cases, Joint Bone Spine, 72, 319–321, https://doi.org/10.1016/j.jbspin.2004.12.005, 2005.
Al-Sayyad, M. J. and Abumunaser, L. A.: Tuberculous arthritis revisited as a forgotten cause of monoarticular arthritis, Ann. Saudi Med., 31, 398–401, https://doi.org/10.4103/0256-4947.83210, 2011.
Bennet, J. E., Dolin, R., and Blaser, M. J. (Eds.): Infectious Arthritis of Native Joints. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 9th Edition, Elsivier Inc, Philadelphia, ISBN 9780323482554, 2020.
Berney, S., Goldstein, M., and Bishko, F.: Clinical and diagnostic features of tuberculous arthritis, Am. J. Med., 53, 36–42, https://doi.org/10.1016/0002-9343(72)90113-1, 1972.
Broderick, C., Hopkins, S., Mack, D. J. F., Aston, W., Pollock, R., Skinner, J. A., and Warren, S.: Delays in the diagnosis and treatment of bone and joint tuberculosis in the United Kingdom, Bone Joint J., 100-b, 119–124, https://doi.org/10.1302/0301-620x.100b1.Bjj-2017-0357.R1, 2018.
Darbyshire, J.: Five-year assessment of controlled trials of short-course chemotherapy regimens of 6, 9 or 18 months' duration for spinal tuberculosis in patients ambulatory from the start or undergoing radical surgery, Fourteenth report of the Medical Research Council Working Party on Tuberculosis of the Spine, Int. Orthop., 23, 73–81, https://doi.org/10.1007/s002640050311, 1999.
Dhillon, M. S. and Nagi, O. N.: Tuberculosis of the foot and ankle, Clin. Orthop. Relat. Res., 398, 107–113, https://doi.org/10.1097/00003086-200205000-00015, 2002.
Dolberg, O. T., Schlaeffer, F., Greene, V. W., and Alkan, M. L.: Extrapulmonary tuberculosis in an immigrant society: clinical and demographic aspects of 92 cases, Rev. Infect Dis., 13, 177–179, https://doi.org/10.1093/clinids/12.5.177, 1991.
Duan, X. J. and Yang, L.: Arthroscopic management for early-stage tuberculosis of the ankle, J. Orthop. Surg. Res., 14, 25, https://doi.org/10.1186/s13018-018-1048-y, 2019.
Gardam, M. and Lim, S.: Mycobacterial osteomyelitis and arthritis, Infect. Dis. Clin. N. Am., 19, 819–830, https://doi.org/10.1016/j.idc.2005.07.008, 2005.
Gu, Y., Wang, G., Dong, W., Li, Y., Ma, Y., Shang, Y., Qin, S., and Huang, H.: Xpert MTB/RIF and GenoType MTBDRplus assays for the rapid diagnosis of bone and joint tuberculosis, Int. J. Infect. Dis., 36, 27–30, https://doi.org/10.1016/j.ijid.2015.05.014, 2015.
Gursu, S., Yildirim, T., Ucpinar, H., Sofu, H., Camurcu, Y., Sahin, V., and Sahin, N.: Long-term Follow-up Results of Foot and Ankle Tuberculosis in Turkey, J. Foot Ankle Surg., 53, 557–561, https://doi.org/10.1053/j.jfas.2014.04.012, 2014.
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., and Schünemann, H. J.: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations, Bmj, 336, 924–926, https://doi.org/10.1136/bmj.39489.470347.AD, 2008.
Habaxi, K. K., Wang, L., Miao, X. G., Alimasi, W. Q., Zhao, X. B., Su, J. G., and Yuan, H.: Total knee arthroplasty treatment of active tuberculosis of the knee: a review of 10 cases, Eur. Rev. Med. Pharmacol. Sci., 18, 3587–3592, 2014.
Hogan, J. I., Hurtado, R. M., and Nelson, S. B.: Mycobacterial Musculoskeletal Infections, Infect. Dis. Clin. N. Am., 31, 369–381, https://doi.org/10.1016/j.idc.2017.01.007, 2017.
Huang, T. Y. Wu, T. S., Yang, C. C., Chiang, P. C., Yu, K. H., and Lee, M. H.: Tuberculous arthritis – A fourteen-year experience at a tertiary teaching hospital in Taiwan, J. Microbiol. Immunol., 40, 493–499, 2007.
Johansen, I. S., Nielsen, S. L., Hove, M., Kehrer, M., Shakar, S., Woyen, A. V. T., Andersen, P. H., Bjerrum, S., Wejse, C., and Andersen, A. B.: Characteristics and Clinical Outcome of Bone and Joint Tuberculosis From 1994 to 2011: A Retrospective Register-based Study in Denmark, Clin. Infect. Dis., 61, 554-562, https://doi.org/10.1093/cid/civ326, 2015.
Kapukaya, A., Subasi, M., Bukte, Y., Gur, A., Tuzuner, T., and Kilnc, N.: Tuberculosis of the shoulder joint, Joint Bone Spine, 73, 177–181, https://doi.org/10.1016/j.jbspin.2005.03.015, 2006.
Kim, S. J., Postigo, R., Koo, S., and Kim, J. H.: Total hip replacement for patients with active tuberculosis of the hip: a systematic review and pooled analysis, Bone Joint J., 95-b, 578–582, https://doi.org/10.1302/0301-620x.95b5.31047, 2013.
Kim, Y. Y., Ahn, J. Y., Sung, Y. B., Ko, C. U., Shim, J. C., Park, H. S., and Bai, G. H.: Long-term results of Charnley low-friction arthroplasty in tuberculosis of the hip, J. Arthroplasty, 16, 106–110, https://doi.org/10.1054/arth.2001.28720, 2001.
Kumar, V., Garg, B., and Malhotra, R.: Total hip replacement for arthritis following tuberculosis of hip, World Journal of Orthopedics, 6, 636–640, https://doi.org/10.5312/wjo.v6.i8.636, 2015.
Lesić, A. R., Pesut, D. P., Marković-Denić, L., Maksimović, J., Cobeljić, G., Milosević, I., Atkinson, H. D., and Bumbasirević, M.: The challenge of osteo-articular tuberculosis in the twenty-first century: a 15-year population-based study, Int. J. Tuberc. Lung Dis., 14, 1181–1186, 2010.
Lewinsohn, D. M., Leonard, M. K., LoBue, P. A., Cohn, D. L., Daley, C. L., Desmond, E., Keane, J., Lewinsohn, D. A., Loeffler, A. M., Mazurek, G. H., O'Brien, R. J., Pai, M., Richeldi, L., Salfinger, M., Shinnick, T. M., Sterling, T. R., Warshauer, D. M., and Woods, G. L.: Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children, Clin. Infect. Dis., 64, 111–115, https://doi.org/10.1093/cid/ciw778, 2017.
Li, Y., Jia, W., Lei, G., Zhao, D., Wang, G., and Qin, S.: Diagnostic efficiency of Xpert MTB/RIF assay for osteoarticular tuberculosis in patients with inflammatory arthritis in China, PLOS ONE, 13, e0198600, https://doi.org/10.1371/journal.pone.0198600, 2018.
Liu, C. S., Liu, F. Z., Wang, X. Y., Yao, Y., Qiao, L., Fu, J., and Wang, D.: Comparison of total curative effect between total hip arthroplasty and hip arthrodesis in treating coxotuberculosis, Eur. Rev. Med. Pharmacol. Sci., 22, 90–95, https://doi.org/10.26355/eurrev_201807_15369, 2018.
Mariconda, M., Cozzolino, A., Attingenti, P., Cozzolino, F., and Milano, C.: Osteoarticular tuberculosis in a developed country, J. Infect., 54, 375–380, https://doi.org/10.1016/j.jinf.2006.06.006, 2007.
Mateo, L., Manzano, J. R., Olivé, A. Manterola, J. M., Pérez, R., Tena, X., and Prats, M.: Tuberculosis osteoarticular: Estudio de 53 casos, Med. Clin., 129, 506–509, https://doi.org/10.1157/13111371, 2007.
Mohanty, M., Mishra, B., Jain, M., and Karaniveed Puthiyapura, L.: Diagnostic role of Xpert-MTB RIF assay in osteoarticular tuberculosis: A retrospective study, World J. Orthop., 13, 289–296, https://doi.org/10.5312/wjo.v13.i3.289, 2022.
Nahid, P., Dorman, S. E., Alipanah, N., Barry, P. M., Brozek, J. L., Cattamanchi, A., Chaisson, L. H., Chaisson, R. E., Daley, C. L., Grzemska, M., Higashi, J. M., Ho, C. S., Hopewell, P. C., Keshavjee, S. A., Lienhardt, C., Menzies, R., Merrifield, C., Narita, M., O'Brien, R., Peloquin, C. A., Raftery, A., Saukkonen, J., Schaaf, H. S., Sotgiu, G., Starke, J. R., Migliori, G. B., and Vernon, A.: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis, Clin. Infect. Dis., 63, e147–e195, https://doi.org/10.1093/cid/ciw376, 2016.
Nahid, P., Mase, S. R., Migliori, G. B., Sotgiu, G., Bothamley, G. H., Brozek, J. L., Cattamanchi, A., Cegielski, J. P., Chen, L., Daley, C. L., Dalton, T. L., Duarte, R., Fregonese, F., Horsburgh Jr., C. R., Ahmad Khan, F., Kheir, F., Lan, Z., Lardizabal, A., Lauzardo, M., Mangan, J. M., Marks, S. M., McKenna, L., Menzies, D., Mitnick, C. D., Nilsen, D. M., Parvez, F., Peloquin, C. A., Raftery, A., Schaaf, H. S., Shah, N. S., Starke, J. R., Wilson, J. W., Wortham, J. M., Chorba, T., and Seaworth, B.: Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline, Am. J. Respir. Crit. Care Med., 200, e93–e142, https://doi.org/10.1164/rccm.201909-1874ST, 2019.
Ozturkmen, Y., Üzümcügil, O., Karamehmetoglu, M., Leblebici, C., and Caniklioglu, M.: Total knee arthroplasty for the management of joint destruction in tuberculous arthritis, Knee Surg. Sports Traumatol. Arthrosc., 22, 1076–1083, https://doi.org/10.1007/s00167-013-2473-4, 2014.
Pigrau-Serrallach, C. and Rodríguez-Pardo, D.: Bone and joint tuberculosis, Eur. Spine J., 22, 556–566, https://doi.org/10.1007/s00586-012-2331-y, 2013.
Qian, Y., Han, Q. X., Liu, W. J., Yuan, W. E., and Fan, C. Y.: Characteristics and management of bone and joint tuberculosis in native and migrant population in Shanghai during 2011 to 2015, Bmc Infect. Dis., 18, 543, https://doi.org/10.1186/s12879-018-3456-3, 2018.
Ravn, C., Neyt, J., Benito, N., Abreu, M. A., Achermann, Y., Bozhkova, S., Coorevits, L., Ferrari, M. C., Gammelsrud, K. W., Gerlach, U.-J., Giannitsioti, E., Gottliebsen, M., Jørgensen, N. P., Madjarevic, T., Marais, L., Menon, A., Moojen, D. J., Pääkkönen, M., Pokorn, M., Pérez-Prieto, D., Renz, N., Saavedra-Lozano, J., Sabater-Martos, M., Sendi, P., Tevell, S., Vogely, C., Soriano, A., and the SANJO guideline group: Guideline for management of septic arthritis in native joints (SANJO), J. Bone Joint Infect., 8, 29–37, https://doi.org/10.5194/jbji-8-29-2023, 2023.
Ren, C., Tang, J., and Xia, L.: Interferon gamma release assays for diagnosis of osteoarticular tuberculosis: A systematic review and meta-analysis, PLoS One, 17, e0269234, https://doi.org/10.1371/journal.pone.0269234, 2022.
Samuel, S., Boopalan, P. R., Alexander, M., Ismavel, R., Varghese, V. D., and Mathai, T.: Tuberculosis of and around the ankle, J. Foot Ankle Surg., 50, 466–472, https://doi.org/10.1053/j.jfas.2011.04.002, 2011.
Sandher, D. S., Al-Jibury, M., Paton, R. W., and Ormerod, L. P.: Bone and joint tuberculosis – Cases in blackburn between 1988 and 2005, J. Bone Joint Surg. Br., 89B, 1379–1381, https://doi.org/10.1302/0301-620x.89b10.18943, 2007.
Shen, H. L., Xia, Y. Y., Li, P., Wang, J., and Han, H.: Arthroscopic operations in knee joint with early-stage tuberculosis, Arch. Orthop. Trauma Surg., 130, 357–361, https://doi.org/10.1007/s00402-009-0881-1, 2010.
Sun, Q., Wang, S., Dong, W., Jiang, G., Huo, F., Ma, Y., Huang, H., and Wang, G.: Diagnostic value of Xpert MTB/RIF Ultra for osteoarticular tuberculosis, J. Infect., 79, 153–158, https://doi.org/10.1016/j.jinf.2019.06.006, 2019.
Tang, K. L., Li, Q. H., Chen, G. X., Guo, L., Dai, G., and Yang, L.: Arthroscopically assisted ankle fusion in patients with end-stage tuberculosis, Arthroscopy, 23, 919–922, https://doi.org/10.1016/j.arthro.2007.04.004, 2007.
Tang, X., Zhu, J., Li, Q., Chen, G., Fu, W. L., and Li, J.: Knee arthrodesis using a unilateral external fixator combined with crossed cannulated screws for the treatment of end-stage tuberculosis of the knee, BMC Musculoskelet. Disord., 16, 197, https://doi.org/10.1186/s12891-015-0667-2, 2015.
Titov, A. G., Nakonechniy, G. D., Santavirta, S., Serdobintzev, M. S., Mazurenko, S. I., and Konttinen, Y. T.: Arthroscopic operations in joint tuberculosis, Knee, 11, 57–62, https://doi.org/10.1016/s0968-0160(03)00035-8, 2004.
Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., Moher, D., Peters, M. D. J., Horsley, T., Weeks, L., Hempel, S., Akl, E. A., Chang, C., McGowan, J., Stewart, L., Hartling, L., Aldcroft, A., Wilson, M. G., Garritty, C., Lewin, S., Godfrey, C. M., Macdonald, M. T., Langlois, E. V., Soares-Weiser, K., Moriarty, J., Clifford, T., Tunçalp, Ö., and Straus, S. E.: PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation, Ann. Intern. Med., 169, 467–473, https://doi.org/10.7326/M18-0850, 2018.
Tuli, S. M.: General principles of osteoarticular tuberculosis, Clin. Orthop. Relat. Res., 398, 11–19, https://doi.org/10.1097/00003086-200205000-00003, 2002.
Venkat, B., Aggarwal, V., Aggarwal, N., and Sharma, S.: Imaging Features of Extraspinal Osteoarticular Tuberculosis and its Mimickers: A Review, Journal of Clinical & Diagnostic Research, 12, 1–7, https://doi.org/10.7860/JCDR/2018/32131.11815, 2018.
Vohra, R., Kang, H. S., Dogra, S., Saggar, R. R., and Sharma, R.: Tuberculous osteomyelitis, J. Bone Joint Surg. Br., 79, 562–566, https://doi.org/10.1302/0301-620x.79b4.7618, 1997.
Wang, G., Yang, X., Zhu, J., Dong, W., Huang, M., Jiang, G., Zhao, L., Qin, S., Chen, X., and Huang, H.: Evaluation of the efficacy of Myco/F lytic system, MGIT960 system and Lowenstein-Jensen medium for recovery of Mycobacterium tuberculosis from sterile body fluids, Sci. Rep., 6, 37757, https://doi.org/10.1038/srep37757, 2016.
Zeng, M., Hu, Y. H., Leng, Y., Xie, J., Wang, L., Li, M. Q., and Zhu, J. X.: Cementless total hip arthroplasty in advanced tuberculosis of the hip, Int. Orthop., 39, 2103–2107, https://doi.org/10.1007/s00264-015-2997-y, 2015.
Zeng, M., Xie, J., Wang, L., and Hu, Y. H.: Total knee arthroplasty in advanced tuberculous arthritis of the knee, Int. Orthop., 40, 1433–1439, https://doi.org/10.1007/s00264-015-3050-x, 2016.
Zhao, J., Pu, D., Zhang, Y., Qu, J., Lu, B., and Cao, B.: Comparison of Performances of GeneXpert MTB/RIF, Bactec MGIT 960, and Bactec Myco/F Systems in Detecting Mycobacterium tuberculosis in Biopsy Tissues: a Retrospective Study, Microbiol. Spectrum, 11, e01414–01422, https://doi.org/10.1128/spectrum.01414-22, 2023.