the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Pyogenic spinal infections warrant a total spine MRI
Cristian Balcescu
Khalid Odeh
Alexander Rosinski
Brandon Nudelman
Adam Schlauch
Ishan Shah
Victor Ungurean Jr.
Priya Prasad
Jeremi Leasure
Flora Stepansky
Amit Piple
Dimitriy Kondrashov
Study design: retrospective case series. Objective: the presenting clinical symptoms of spinal infections are often nonspecific and a delay in diagnosis can lead to adverse patient outcomes. The morbidity and mortality of patients with multifocal spinal infections is significantly higher compared to unifocal infections. The purpose of the current study was to analyse the risk factors for multifocal spinal infections. Methods: we conducted a retrospective review of all pyogenic non-tuberculous spinal infections treated surgically at a single tertiary care medical center from 2006–2020. The medical records, imaging studies, and laboratory data of 43 patients during this time period were reviewed and analysed after receiving Institutional Review Board approval. Univariate and multivariate analyses were performed to identify factors associated with a multifocal spinal infection. Results: 15 patients (35 %) had multifocal infections. In univariate analysis, there was a significant association with chronic kidney disease (p=0.040), gender (p=0.003), a white blood cell count (p=0.011), and cervical (p<0.001) or thoracic (p<0.001) involvement. In multivariate analysis, both cervical and thoracic involvement remained statistically significant (p=0.001 and p<0.001, respectively). Conclusions: patients with infections in the thoracic or cervical region are more likely to have a multifocal infection. Multifocal pyogenic spinal infections remain a common entity and a total spine MRI should be performed to aid in prompt diagnosis.
- Article
(1216 KB) - Full-text XML
- BibTeX
- EndNote
Pyogenic spinal infections comprise a wide range of clinical entities and include infections of the disc, vertebra, facet joints, dura, spinal cord, and paravertebral soft tissues (Hadjipavlou et al., 2000). The mechanism of infection is most commonly by a hematogenous spread with inoculation of the vertebral endplates (Lew and Waldvogel, 2004). The clinical consequences can be devastating, and this condition can lead to irreversible neurologic damage or even death (Doutchi et al., 2015). While the presenting clinical symptoms of spinal infection are often nonspecific and can be confused at times with those of a degenerative condition, a prompt diagnosis of all affected regions of the spine is critical to improving neurological and functional outcomes (McHenry et al., 2002).
On initial presentation, patients with a pyogenic spinal infection may present with an axial neck or back pain, radiculopathy, myelopathy, or even paralysis (Lew and Waldvogel, 2004). A multitude of patient-related risk factors have been associated with spinal infections. Quite often the patients have a compromised immune system due to a variety of causes. These include an advanced age, chronic steroid usage, diabetes mellitus, human immunodeficiency virus (HIV), an immunocompromised state, a presence of intravascular devices, orthopaedic hardware, intravenous drug abuse, malignancy, malnutrition, recent spinal surgery, autoimmune condition, renal failure, and septicemia (Gasbarrini et al., 2005; Reihsaus et al., 2000; Sampath and Rigamonti, 1999). Magnetic resonance imaging (MRI) is the most reliable imaging modality for detecting spinal infections, with a sensitivity and specificity greater than 90 % (Modic et al., 1985; Ledermann et al., 2003). The American College of Radiology guidelines state that emergent MRI of the spine is indicated in patients with new or worsening neurologic deficits, and either signs or symptoms of a spine infection (Lavi et al., 2018).
While the algorithm for diagnosing unifocal spinal infections is relatively straightforward, there is no consensus on the approach toward screening and excluding multifocal spinal infections.
Multifocal spinal infections are defined as infections located in greater than one region of the spine (Butler et al., 2006; Korovessis et al., 2012; Chow et al., 1996; Deshmukh, 2010). Those can be either contiguous or non-contiguous and separated by one or more healthy segments. The multifocal spine infections may be present at the same time (synchronous) or present at different points in time (metachronous).
The morbidity and mortality of patients with multifocal spinal infections is significantly higher compared to unifocal infections (Butler et al., 2006; Korovessis et al., 2012; Chow et al., 1996; Deshmukh, 2010). Therefore, it is imperative to promptly identify all involved regions of the spine so that the appropriate treatment can be administered. Our previous single-institution retrospective study found an incidence of 35 % for multifocal infections in the patients surgically treated for non-tuberculous pyogenic spinal infections (Balcescu et al., 2019). Other studies have reported incidences of multifocal spinal infection ranging from 4 % to 30 % (Ledermann et al., 2003; Mann et al., 2004; Ziu et al., 2014; Cox et al., 2018).
The screening practices for multifocal spinal infections vary significantly across institutions and countries. In some geographic regions, it is a routine practice to obtain a multisequence sagittal MRI of the entire spine (Cox et al., 2018). However, in other regions, only a limited part of the spine is typically imaged during the examination. This limited imaging may be attributed to the overly aggressive medico-legal systems, increased economic cost, loss of potential reimbursement for the future spine examinations, and an increased time in the scanner. Few studies have investigated the specific predictors of multifocal spinal infections (Siam et al., 2013; Abbara et al., 2016; Siam, 2016) when compared to unifocal infections (Hadjipavlou et al., 2000; Butler et al., 2006; Korovessis et al., 2012; Mann et al., 2004; Malawski and Lukawski, 1991). Elucidation of these differences may provide clarity and standardisation in the screening for multifocal infections. Our previous study had investigated those differences and found that patients surgically treated for cervical or thoracic spinal infections had a high rate of multifocal spinal infections (71 % and 83 %, respectively) (Balcescu et al., 2019). However, the power of our study was small due to the sole inclusion of patients who were operated on at a single institution over the course of 6 years.
The purpose of the current study was to revisit the risk factors for multifocal spinal infections while using a more robust data set. Our results may promote a prompt diagnosis of the occult multifocal spinal infections and improve patient outcomes (Redekop and Maestro, 1992; Verner and Musher, 1985).
The vascular anatomy of each spinal region varies significantly. The prevertebral pharyngeal venous plexus may permit a bacterial spread between the head and neck and cervical spine (Wiley and Trueta, 1959). The Batson's paravertebral venous plexus may allow for the bacterial spread between pelvic organs and the lumbar spine (Batson, 1967). Based on the vascular anatomic differences and the presumed differences in the ease of infectious spread, we have hypothesised that the initial region of spinal involvement is a significant predictor of multifocality.
We conducted a retrospective review of all pyogenic non-tuberculous spinal infections treated surgically by fellowship-trained spine surgeons at our institution from 1 April 2006 to 30 April 2020. Preliminary screening was performed using International Classification of Disease (ICD) 9 and 10 codes to search the electronic medical record for all potential patients. Different types of pyogenic spinal infections were considered, such as vertebral osteomyelitis, discitis, epidural abscess, and septic facet arthritis. The diagnostic criteria included characteristic MRI findings (e.g. hypointense signal on T1-weighted images, hyper-intense signal on T2-weighted images) and suggestive laboratory results (e.g. elevated white blood cell count (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and lactate). The final diagnosis was based on clinical, radiological, and serologic evidence. Exclusion criteria were adolescents aged under 18 years and early postoperative infections (within 6 months of a previous spine operation). The human subjects review committee at our institution provided approval for this study.
Data were collected regarding demographics (age, sex, race), patient characteristics (weight, height, body mass index), predisposing factors (past medical history, social history), preoperative laboratory data (WBC, ESR, CRP, lactate), the indications for surgery, level of spinal involvement, bacteriology results (blood cultures, spinal tissue cultures), MRI/CT imaging, therapeutic management, and duration of hospitalisation. Immunocompromised status, defined as a history of diabetes mellitus, rheumatoid arthritis, cancer, or malnutrition, was also assessed. Recent infection was defined as a history of infection within 3 months of presentation of spinal infection. Albumin <3.5 was used to define a state of malnutrition. A fellowship-trained musculoskeletal or neuroradiologist reviewed each of the imaging studies and determined the presence of prevertebral soft tissue component involvement, epidural soft tissue component involvement, and T1 abnormality. All patients included in the study received the following seven MRI sequences: (1) sagittal T1-weighted; (2) sagittal T2-weighted fast relaxation fast spin echo (FRFSE); (3) sagittal short tau inversion recovery (STIR); (4) axial T2-weighted FRFSE; (5) axial T1-weight; (6) axial T1-weighted fast spin echo (FSE); and (7) sagittal T1-weighted FSE.
Patients with pyogenic infections involving multiple regions of the spinal column (e.g. cervical, thoracic, lumbar) were allocated to the “multifocal infection” group, including infections that were contiguous across regions (e.g. an epidural abscess spanning across the thoracolumbar junction). Patients in the “unifocal infection” group had infections involving one spinal region only, although multiple anatomical areas may have been involved (e.g. noncontiguous L2–3 epidural abscess plus L4–5 discitis).
Statistical analysis
Descriptive statistics, including the mean, variance, and standard deviation, were calculated for continuous variables. Categorical variables were summarised with frequencies and proportions. Logistic regression and t-tests were performed using JMP ver. 11.0.0 (SAS Institute Inc., Cary, NC, USA). Regression coefficients were obtained for each independent variable when all other variables were held constant. Chi-square tests and t-tests with Tukey–Kramer correction were used to identify statistically significant differences between the multifocal and unifocal spinal infection groups. Variables trending toward significance or that were significantly associated with multifocal infection were identified in a univariate logistic regression analysis and alpha equal to 0.10. These variables were then entered into a multivariate logistic model to identify individual risk factors. An alpha equal to 0.05 was used as the significance level when evaluating multivariate significance tests.
Table 1Summary data and comparisons of continuous variables collected during the study. Significance between unifocal and multifocal infection subjects are indicated with an asterisk (*).
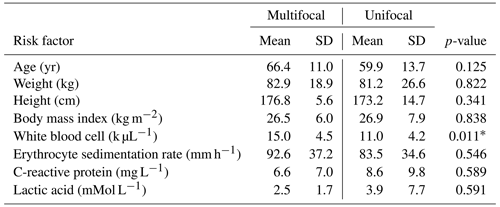
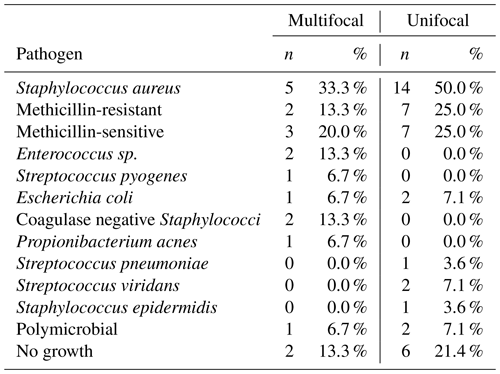
In total, 43 patients were identified and included in this study, of which, 15 (34.9 %) were diagnosed with multifocal infection and 28 (65.1 %) were diagnosed with unifocal infection. The average age of the total cohort was 62.1±12.9 years and was composed of 34 (79.0 %) male patients. There were no statistically significant differences in mean age, weight, height, body mass index, ESR, CRP, or lactate between the unifocal and multifocal infection groups (p>0.05 for all comparisons), as displayed in Table 1. All 43 patients underwent bacteriology studies consisting of blood culture or tissue culture. In total, 35 (81.4 %) patients were positive for bacterial growth on culture, with Staphylococcus aureus being the most commonly identified pathogen. A summary of all pathogens identified in the unifocal and multifocal cohorts is depicted in Table 2.
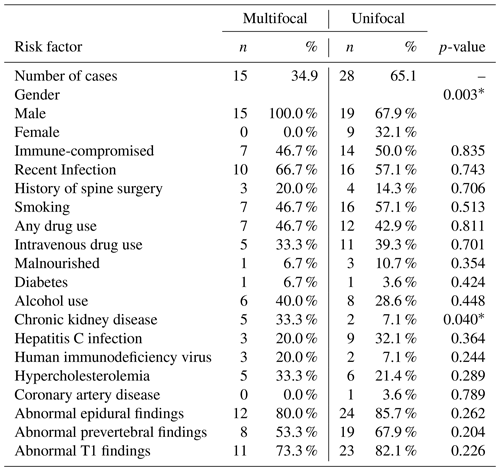
Table 3Summary data and comparisons of categorical variables collected during the study. Significance between unifocal and multifocal infection subjects are indicated with an asterisk (*).
3.1 Comorbidities
There were no statistically significant differences in immunocompromised state, antibiotic use, history of spine surgery, smoking, intravenous drug use, malnourished state, diabetes, alcohol use, hepatitis C infection, HIV, hypercholesterolemia, or coronary artery disease between the unifocal and multifocal infection groups (p>0.05 for all comparisons). Categorical variables are displayed in Table 3.
3.2 Imaging
Rates of abnormal epidural findings on MRI, abnormal prevertebral findings, and abnormal T1 findings were also not significantly different between the two groups (p>0.05 for all comparisons).
3.3 Univariate analysis
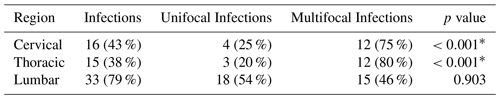
Several outcome measures were significantly associated with multifocal infection on univariate analysis. There was an association with gender (p=0.011); unifocal infections were recorded for all nine of our female patients. White blood cell count was associated (p=0.011) with multifocal infections. Unifocal infection patients had an average leukocyte count of 10.9±4.2 k µL−1 compared to multifocal with 15.0±4.4 k µL−1. Chronic kidney disease was associated with multifocal infection (p=0.040). Two patients with chronic kidney disease presented with a unifocal infection and five patients presented with multifocal infection. The presence of a multifocal infection was significantly associated with both cervical (p<0.001) and thoracic (p<0.001) involvement. The presence of a lumbar infection was not significantly associated (p=0.903) with multifocal infection. Among the patients in our study, 35 % had cervical infections, 30 % had thoracic infections, and 85 % had lumbar infections, as displayed in Table 4.
3.4 Multivariate analysis
Cervical and thoracic involvement remained statistically significant in a multivariate analysis (p=0.001 and p<0.001, respectively). No statistically significant association was observed with either gender or white blood cell count (p=0.080 and p=0.999, respectively) in our multivariate analysis.
3.5 Illustrative case
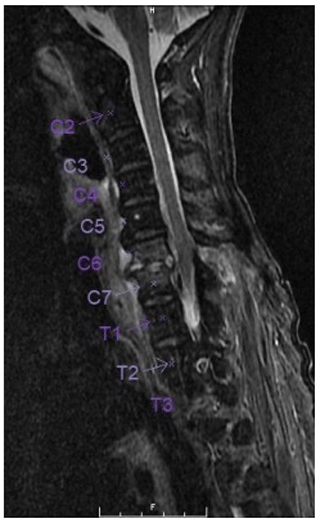
A 67-year-old Asian homeless woman with a history of delusional psychosis was admitted to the hospital with urosepsis secondary to E. coli urinary tract infection (UTI), bacteremia, and sternoclavicular osteomyelitis. She underwent a 6-week course of intravenous antibiotics; however, her inflammatory markers remained persistently elevated. She had developed severe neck and lower back pain, which warranted a full spine MRI. Although she had antigravity strength in all four extremities, the degree of any neurologic deficits was difficult to assess due to her underlying psychiatric illness. Cervical spine MRI demonstrated spondylodiscitis at C6–7 with a prevertebral collection as well as a retrovertebral epidural abscess severely compressing the spinal cord (Fig. 1). There was no evidence of myelomalacia. Bone edema in both C6 and C7 vertebrae involved the entire motion segment. The combination of spondylodiscitis and epidural abscess resulted in a severe central canal stenosis and spinal cord compression. The lumbar spine MRI demonstrated L2–3 spondylodiscitis resulting in moderate-to-severe central canal stenosis with perivertebral extension. There also did appear to be infectious myositis of both the erector spinae musculature as well as bilateral iliopsoas. There was a right-sided iliopsoas abscess at the level of the L2–3 disc. There was also a severe bone destruction involving the inferior part of L2 vertebra (Figs. 2, 3).
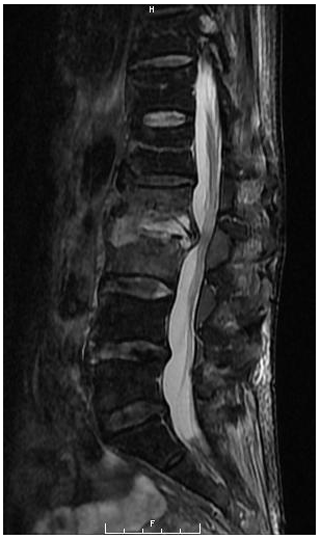
Figure 2Sagittal T2-weighted MRI of the lumbar spine demonstrating L2–3 spondylodiscitis with moderate to severe spinal canal stenosis. Severe bone destruction was noted at L2.
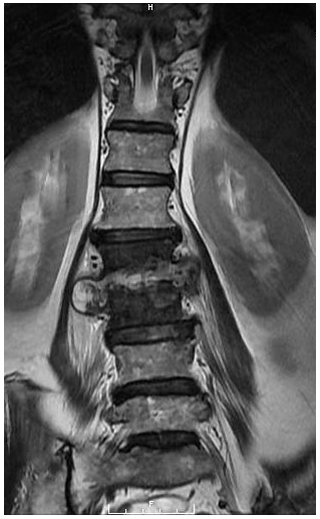
Figure 3Coronal T2-weighted MRI of the lumbar spine demonstrating L2–3 spondylodiscitis 429 with infectious myositis of the bilateral erector spinae musculature and iliopsoas musculature. A right iliopsoas abscess was also noted.
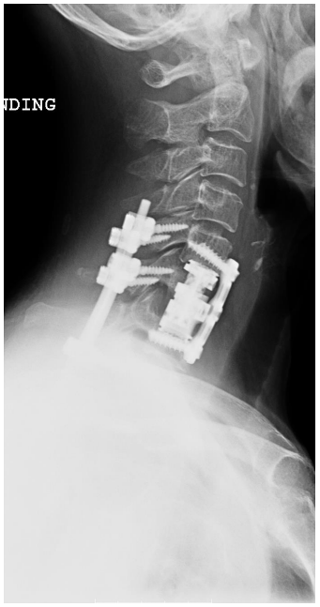
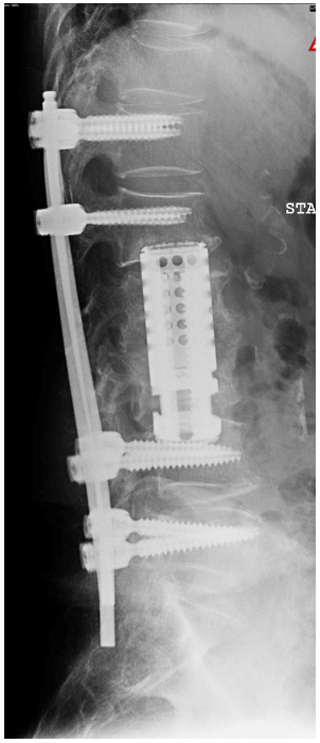
She underwent a staged debridement and spondylectomy of the infected levels followed by reconstruction of the corpectomy defects were reconstructed with expandable cages. The stability was restored by a long fusion construct using pedicle screws and rods in the lumbar spine as well as lateral mass screws and pedicle screws with rods across the cervicothoracic junction (Figs. 4, 5).
She also underwent a second 6-week course of IV antibiotics. Following the surgery, the patient had a gradual resolution of her pain, regained the ability to walk, and had a normalisation of the inflammatory markers. This was despite of extensive use of hardware in the midst of spine infection.
The current study has reconfirmed that patients presenting with either the thoracic or cervical spinal infections are highly likely to have a multifocal infection. There were no statistically significant differences between the unifocal and multifocal groups other than an increased risk of multifocality when the cervical and/or thoracic regions were involved. The percentage of multifocal infections was consistent with our previous study (35 %) and was slightly higher than the range reported in the literature.
While the diagnosis of spinal infections may be difficult, the consequences of a delayed diagnosis and untreated infections are severe. Recent published studies have shown a diagnosis of a spinal epidural abscess to be associated with mortality rates as high as 37.5 % following surgical treatment, and permanent neurologic deficits as high as 30 %–50 % (Du et al., 2019). The morbidity also differs depending on the spinal region involved. Hadjipavlou et al. (2000) found that epidural abscesses in the cervical and thoracic spine have a significantly higher risk of a severe neurological deficit compared to the lumbar spine (Hadjipavlou et al., 2000). This phenomenon might be explained by the less tolerance of the spinal cord to compression as opposed to the cauda equina. Another possible explanation is the potential for a thrombosis of the anterior spinal artery with the abscesses at the spinal cord level (Richardson and Wattenbarger, 2021; van de Warrenburg et al., 2004).
Therefore, we believe that obtaining an MR imaging of the entire spine as a screening tool in patients with a suspected spinal infection increases the yield of discovery of additional sites of infection. This may change the course of management from a medical to a surgical treatment strategy and result in the significantly decreased morbidity and mortality. This change in the treatment strategy with multifocal involvement is not dissimilar to polytrauma patients, when an otherwise non-operative spine fracture may become operative in the setting of accompanying extremity fractures (McLain and Benson, 1999).
In a similar previous study, Cox et al. (2018) found that upon a sagittal MR imaging of the entire spine in patients with a known unifocal non-tuberculous spondylodiscitis, 23 % of patients had additional sites of spinal infection at distant sites and 58 % of these patients underwent surgical management. Their protocol for patients with a clinical single-level spondylodiscitis includes a multi-sequence sagittal MRI of the entire spine (Cox et al., 2018). Our study adds additional data to support this intuitive recommendation.
While pan-spine sagittal images are a routine practice in some countries, it is not uncommon for institutions in the United States to limit the imaging to a single region of the spine during the examination. This may partly be explained by prior studies with conflicting results compared to ours. In a retrospective study of 91 patients with tuberculous (TBS) and pyogenic spondylodiscitis (PS), Abbara et al. (2016) reported that 33 % of TBS cases were multifocal, whereas in the PS group they were all unifocal. The study concluded that the MR imaging of the entire spine is only necessary in TBS cases and not recommended for PS cases. Limited imaging practices may also be attributed to the drive to decrease institutional costs as well as the time in the scanner and trying to prevent malpractice suits against radiologists who are liable for a significant pathology that is missed in the incompletely visualised regions of the spine. Certain characteristics on MR imaging may also predict the risk of multifocal spinal infections. The presence of osteomyelitis and paravertebral abscesses on imaging have been thought to be associated with both increased infection severity and multifocality. However, our study found no significant differences in the MRI characteristics between the unifocal and multifocal cohorts. In our study, the MR imaging suggested that the majority of multifocal spinal infection had a thoracic or cervical component. This is similar to a previous study by Abdelrahman et al. (2013) looking at 1138 consecutive cases of spondylitis and spondylodiscitis over 16 years. They reported that 6.8 % of these cases showed additional non-contiguous sites of spinal infection and that the most common patterns of involvement were thoracic to lumbar (43 %), lumbar to lumbar (16 %), and cervical to lumbar (14 %).
This study adds valuable data to the existing spine infection literature as it demonstrates the highest incidence of multifocal spinal infections reported thus far. This may be attributed to the challenging patient population that our urban tertiary referral center treats, which frequently includes drug users and homeless patients. This may limit the generalisability of our results. Patients within our study were also subjected to a relatively uniform evaluation, diagnosis, and treatment, since this was a single-institution study. Additionally, this is the first study to evaluate several risk factors unique to multifocal spinal infections when compared to unifocal spinal infections, including patient characteristics, type of infection, levels of involvement, laboratory results, bacterial cultures, and MRI features.
Limitations of this study include its retrospective nature and a relatively small cohort size. The present study follows our previous study which had a smaller cohort size of 20. We hoped to address the concerns of a small power in our previous study by doubling the cohort size to detect the more subtle differences. We also have excluded the patients with a spinal infection who were treated nonoperatively. The indications for conservative management included an absence of a neurological deficit, an absence of spinal instability, and an absence of intractable pain. Those patients were usually treated with antibiotic therapy, immobilisation (bed rest or bracing), and/or a CT-guided percutaneous drainage. Lastly, we have excluded the patients with a granulomatous spinal infection due to either Mycobacterium tuberculosis, Brucella, or fungal species, as the multifocal presentation associated with those organisms is well documented.
Our study reaffirms our previous conclusion that multifocal spine infections are a common entity especially in patients who present with cervical or thoracic spine involvement. For patients that require operative intervention for a pyogenic spinal infection, a pan-spine MRI is warranted to evaluate for the presence of a multifocal infection.
Because the data contains possible sensitive personal health information we are limited by our institution's policy and it cannot be made available.
CB's contributions included conception, writing, design, analysis, and supervision. KO's contributions included writing, analysis, design, and supervision. AR's contributions included literature review, and data collection and processing. BN's contributions included critical reviews, design, and interpretation. AS's contributions included critical reviews and interpretation. IS's contributions included literature review and critical reviews. VU Jr.'s contributions included analysis, revisions, data collection and processing, and writing. PP's contributions included data collection and processing. JL's contributions included supervision and study design. FS supervision, conception, and study design. AP's contributions include writing, revisions, data analysis, and literature review. DK's contributions included supervision, conception, study design, and critical review.
Dimitriy Kondrashov receives grants/research support from SI-BONE (USD 30 000–50 000), SpineArt (USD 10 000–15 000), and the AO Foundation (USD 40 000–60 000). Jeremi Leasure and Dimitriy Kondrashov receive USD 10 000–15 000 in royalties from SpineArt. For the remaining authors, none were declared.
The research was conducted in accordance with the principles embodied in the Declaration of Helsinki and in accordance with local statutory requirements. This is a retrospective study involving record/imaging review, therefore written consent to participate in the study was waived. All patient anonymity is protected.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was edited by Irene Karharina Sigmund and reviewed by two anonymous referees.
Abbara, A., Tivey, A., John, L., and Davidson, R. N.: Whole spine imaging is justified in tuberculous spondylodiscitis but not pyogenic spondylodiscitis, J. Infect., 72, 125–126, https://doi.org/10.1016/j.jinf.2015.09.034, 2016.
Balcescu, C., Odeh, K., Rosinski, A., Wang, J., Prasad, P., Leasure, J., Ungurean, V., and Kondrashov, D.: High Prevalence of Multifocal Spine Infections Involving the Cervical and Thoracic Regions: A Case for Imaging the Entire Spine, Neurospine, 16, 756–763, https://doi.org/10.14245/ns.1836296.148, 2019.
Batson, O. V.: The vertebral system of veins as a means for cancer dissemination, Progress in Clinical Cancer, 3, 1–18, 1967.
Butler, J. S., Shelly, M. J., Timlin, M., Powderly, W. G., and O'Byrne, J. M.: Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center, Spine, 31, 2695–2700, https://doi.org/10.1097/01.brs.0000244662.78725.37, 2006.
Chow, G. H., Gebhard, J. S., and Brown, C. W.: Multifocal metachronous epidural abscesses of the spine. A case report, Spine, 21, 1094–1097, https://doi.org/10.1097/00007632-199605010-00021, 1996.
Cox, M., Curtis, B., Patel, M., Babatunde, V., and Flanders, A. E.: Utility of sagittal MR imaging of the whole spine in cases of known or suspected single-level spinal infection: Overkill or good clinical practice?, Clin. Imag., 51, 98–103, https://doi.org/10.1016/j.clinimag.2018.02.009, 2018.
Deshmukh V. R.: Midline trough corpectomies for the evacuation of an extensive ventral cervical and upper thoracic spinal epidural abscess, J. Neurosurg., 13, 229–233, https://doi.org/10.3171/2010.3.SPINE09589, 2010.
Doutchi, M., Seng, P., Menard, A., Meddeb, L., Adetchessi, T., Fuentes, S., Dufour, H., and Stein, A.: Changing trends in the epidemiology of vertebral osteomyelitis in Marseille, France, New Microbes and New Infections, 7, 1–7, https://doi.org/10.1016/j.nmni.2015.04.008, 2015.
Du, J. Y., Schell, A. J., Kim, C. Y., Trivedi, N. N., Ahn, U. M., and Ahn, N. U.: 30-day Mortality Following Surgery for Spinal Epidural Abscess: Incidence, Risk Factors, Predictive Algorithm, and Associated Complications, Spine, 44, E500–E509, https://doi.org/10.1097/BRS.0000000000002875, 2019.
Gasbarrini, A. L., Bertoldi, E., Mazzetti, M., Fini, L., Terzi, S., Gonella, F., Mirabile, L., Barbanti Bròdano, G., Furno, A., Gasbarrini, A., and Boriani, S.: Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis, European Review for Medical and Pharmacological Sciences, 9, 53–66, 2005.
Hadjipavlou, A. G., Mader, J. T., Necessary, J. T., and Muffoletto, A. J.: Hematogenous pyogenic spinal infections and their surgical management, Spine, 25, 1668–1679, https://doi.org/10.1097/00007632-200007010-00010, 2000.
Korovessis, P., Repantis, T., and Hadjipavlou, A. G.: Hematogenous pyogenic spinal infection: current perceptions, Orthopedics, 35, 885–892, https://doi.org/10.3928/01477447-20120919-11, 2012.
Lavi, E. S., Pal, A., Bleicher, D., Kang, K., and Sidani, C.: MR Imaging of the Spine: Urgent and Emergent Indications, Seminars in ultrasound, CT, and MR, 39, 551–569, https://doi.org/10.1053/j.sult.2018.10.006, 2018.
Ledermann, H. P., Schweitzer, M. E., Morrison, W. B., and Carrino, J. A.: MR imaging findings in spinal infections: rules or myths?, Radiology, 228, 506–514, https://doi.org/10.1148/radiol.2282020752, 2003.
Lew, D. P. and Waldvogel, F. A.: Osteomyelitis, Lancet, 364, 369–379, https://doi.org/10.1016/S0140-6736(04)16727-5, 2004.
Malawski, S. K. and Lukawski, S.: Pyogenic infection of the spine, Clin. Orthop. Relat. R., 272, 58–66, 1991.
Mann, S., Schütze, M., Sola, S., and Piek, J.: Nonspecific pyogenic spondylodiscitis: clinical manifestations, surgical treatment, and outcome in 24 patients, Neurosurg. Focus, 17, 1–7, https://doi.org/10.3171/foc.2004.17.6.3, 2004.
McHenry, M. C., Easley, K. A., and Locker, G. A.: Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals, Clin. Infect. Dis., 34, 1342–1350, https://doi.org/10.1086/340102, 2002.
McLain, R. F. and Benson, D. R.: Urgent surgical stabilization of spinal fractures in polytrauma patients, Spine, 24, 1646–1654, https://doi.org/10.1097/00007632-199908150-00005, 1999.
Modic, M. T., Feiglin, D. H., Piraino, D. W., Boumphrey, F., Weinstein, M. A., Duchesneau, P. M., and Rehm, S.: Vertebral osteomyelitis: assessment using MR, Radiology, 157, 157–166, https://doi.org/10.1148/radiology.157.1.3875878, 1985.
Redekop, G. J. and Del Maestro, R. F.: Diagnosis and management of spinal epidural abscess, Can. J. Infect. Dis. Med., 19, 180–187, 1992.
Reihsaus, E., Waldbaur, H., and Seeling, W.: Spinal epidural abscess: a meta-analysis of 915 patients, Neurosurg. Rev., 23, 175–205, https://doi.org/10.1007/pl00011954, 2000.
Richardson, C. and Wattenbarger, S.: A case report of quadriplegia and acute stroke from tracking retropharyngeal and epidural abscess complicated by necrotizing fasciitis, JACEP-J. Am. Coll. Emer., 2, e12524, https://doi.org/10.1002/emp2.12524, 2021.
Sampath, P. and Rigamonti, D.: Spinal epidural abscess: a review of epidemiology, diagnosis, and treatment, J. Spinal Disord., 12, 89–93, 1999.
Siam, A., Abdelrahman, H., Allouch, H., and Boehm, H.: Multi-level non-contiguous spinal infections – Series of 77 Cases in a single institution, Eur. Spine J., 22, 11, https://doi.org/10.13140/RG.2.1.2064.6162, 2013.
Siam, A. E.: Whole spine MRI should be recommended for pyogenic spondylodiscitis; response to Abbara et al., J. Infect., 72, 631, https://doi.org/10.1016/j.jinf.2016.03.001, 2016.
van de Warrenburg, B. P., Wesseling, P., Leyten, Q. H., and Boerman, R. H.: Myelopathy due to spinal epidural abscess without cord compression: a diagnostic pitfall, Clin. Neuropathol., 23, 102–106, 2004.
Verner, E. F. and Musher, D. M.: Spinal epidural abscess, Med. Clin. N. Am., 69, 375–384, https://doi.org/10.1016/s0025-7125(16)31049-5, 1985.
Wiley, A. M. and Trueta, J.: The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis, J. Bone Joint Surg., 41-B, 796–809, https://doi.org/10.1302/0301-620X.41B4.796, 1959.
Ziu, M., Dengler, B., Cordell, D., and Bartanusz, V.: Diagnosis and management of primary pyogenic spinal infections in intravenous recreational drug users, Neurosurg. Focus, 37, E3, https://doi.org/10.3171/2014.6.FOCUS14148, 2014.