the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The concordance between preoperative synovial fluid culture and intraoperative tissue cultures in periprosthetic joint infection: a systematic review
Thomas J. A. van Schaik
Lex D. de Jong
Maurits P. A. van Meer
Jon H. M. Goosen
Matthijs P. Somford
Background: this systematic review aims to evaluate the concordance between preoperative synovial fluid culture and intraoperative tissue cultures in patients with periprosthetic joint infection (PJI) undergoing total hip (THA) or knee arthroplasty (TKA) revision surgery. Methods: this review was conducted in accordance with the preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA) statement. Cochrane, Embase, PubMed, and Web of Science databases were searched to identify studies involving patients who had THA or TKA revision surgery for PJI and for whom preoperative synovial fluid culture and intraoperative tissue cultures were performed. Studies were only included if the diagnosis of PJI was based on the EBJIS (the European Bone and Joint Infection Society) or MSIS (Musculoskeletal Infection Society) criteria. Risk of bias was assessed using an amended version of Joanna Briggs Institute's (JBI) critical appraisal checklist for case series. Results: seven studies were included in this review comprising 1677 patients. All studies had a retrospective study design and five studies explored patients undergoing revision surgery of THA or TKA. Concordance rates varied between 52 % and 79 %, but different authors defined and calculated concordance differently. Six studies were judged as having an unclear to high risk of bias and one study as having a low risk of bias. Conclusions: the included studies showed a wide range of concordance rates between preoperative synovial fluid culture and intraoperative tissue cultures and the majority of studies had a high risk of bias. Higher-quality studies are warranted to obtain a more accurate estimate of this concordance rate. We recommend continuing the use of a system such as the EBJIS definition or MSIS criteria when diagnosing PJI.
- Article
(1717 KB) - Full-text XML
-
Supplement
(672 KB) - BibTeX
- EndNote
While the incidence of periprosthetic joint infection (PJI) is increasing worldwide, diagnosing PJI remains challenging because there is no robust single diagnostic test for PJI (Ahmad et al., 2016; Fernández-Sampedro et al., 2017). To date, the validated, evidence-based 2018 International Consensus Meeting (ICM) modified Musculoskeletal Infection Society (MSIS) definition has been commonly used to diagnose PJI (Parvizi et al., 2018). The European Bone and Joint Infection Society (EBJIS) has recently recommended a novel definition set and guidance for PJI which has been supported by the MSIS and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Implant-Associated Infections (ESGIAI) (McNally et al., 2021). The diagnosis of PJI is based on a combination of clinical findings, laboratory results from peripheral blood and synovial fluid, microbiological culture, histological evaluation of periprosthetic tissue, and intraoperative findings. Within the EBJIS criteria, the intraoperatively collected tissue cultures remain the cornerstone in the diagnosis of PJI because these have superior diagnostic sensitivity (65 %–94 %) (Atkins et al., 1998; Spangehl et al., 1999; Pandey et al., 2000; Kheir et al., 2018) compared to the preoperative synovial fluid cultures (45 %–75 %) (Tande and Patel, 2014; Trampuz et al., 2004). As such, the latter are merely recognized as a supportive modality (Osmon et al., 2013).
However, in daily clinical practice, treatment strategies often depend on the preoperative synovial fluid simply because the final results of the intraoperative collected tissue cultures are available only multiple days after surgery. This approach is not a problem if the bacteria found intraoperatively are concordant with those found preoperatively. However, in the case of discordance between preoperative and intraoperative culture results, the antibiotic treatment initiated after preoperative synovial fluid analysis may not always be appropriate. For example, previous studies (Wouthuyzen-Bakker et al., 2019; Lora-Tamayo et al., 2013; Vilchez et al., 2011) have shown that patients who were diagnosed with PJI caused by Staphylococcus aureus, and subsequently treated using a debridement, antibiotics, and implant retention (DAIR) procedure, tended to have worse postoperative outcomes in terms of prosthesis failure compared to patients who had a DAIR following PJI caused by Streptococci. In cases of infection with S. aureus, an orthopedic surgeon might prefer to perform revision surgery instead of a DAIR. This example of the management of acute PJI shows that a lack of concordance between the preoperative synovial fluid culture and intraoperative tissue cultures can lead to suboptimal treatment and may cause the patient harm and/or unnecessary treatment delay. The same may be true for patients with a chronic PJI who are being treated with revision surgery. To date, some studies which have analyzed the concordance in results between preoperative aspiration and intraoperative synovial fluid cultures reported a wide range of 52 % to 78 % (Holleyman et al., 2016; Matter-Parrat et al., 2017; Declercq et al., 2020; Li et al., 2021; Boyle et al., 2021; Schulz et al., 2021).
To our knowledge, no systematic review has been conducted in this area previously. To be able to better guide the orthopedic surgeons' decisions regarding the treatment of patients with PJI, we set out to evaluate the concordance of preoperative synovial fluid culture and intraoperative tissue cultures in patients undergoing revision surgery of their total hip (THA) or knee arthroplasty (TKA).
Prior to data extraction, the protocol for this review was registered in the prospective register of systematic reviews (PROSPERO; registration number CRD42022302223). The review was conducted according to the Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA) guidelines (McInnes et al., 2018).
2.1 Criteria for considering studies for this review
We considered all studies that assessed patients with established PJI who were planned for one-stage or two-stage revision surgery of their THA or TKA, and for whom both preoperative synovial fluid cultures and intraoperative tissue cultures were performed. These studies were required to report on the concordance of the microbiology results between synovial fluid and tissue cultures in terms of percentage agreement, agreement in the types of bacteria found, or both. All studies and case reports published in the English, Dutch, or German language with their full-texts available were considered. We excluded studies that performed the preoperative aspiration after the first surgical intervention of a two-stage revision procedure or if less than two tissue cultures were collected intraoperatively.
2.2 Search methods for identification of studies
First, one review author (Thomas J. A. van Schaik) developed a search strategy (Table S1) which was used to systematically conduct a search in the Cochrane, Embase, PubMed, and Web of Science bibliographic databases from inception up to 1 February 2022. Together with a second review author (Lex D. de Jong), all titles and abstracts of all identified records were independently screened using the web-based systematic reviewing platform Rayyan (Ouzzani et al., 2016). Studies deemed eligible for inclusion, as well as studies where authors were unsure or disagreed about eligibility, were retrieved full-text for further review.
2.3 Critical appraisal of studies
Risk of bias of the included studies was assessed using Joanna Briggs Institute's (JBI's) critical appraisal checklist for case series (Joanna Briggs Institute, 2017). This tool is suitable for critical appraisal of studies lacking a control group and studying patients with a certain disease (in our case PJI) or disease-related outcome (in our case preoperative synovial fluid cultures and intraoperative tissue cultures). An amended version of this critical appraisal checklist was composed using tailored judgment criteria that better suited the context of this review (Table S2), and was first pretested independently by two review authors using two of the identified studies. Subsequently, some items were refined further for clarity. For example, to judge whether valid methods were used for identification of PJI for all patients included in a study (Table S2, item 3), we required the diagnosis to be based on the EBJIS or MSIS criteria. We also only considered a description of clinical patient characteristics as fully sufficient if information regarding “antibiotic use” and the “time between preoperative and intraoperative culture” (Table S2, item 7) was present because these characteristics were deemed to potentially have an influence on the microbiological test results. Using this checklist, the two review authors independently judged the overall risk of bias of each individual study based on the overall number of risk of bias item scores. Disagreements in scoring were resolved by discussion until consensus was reached, and remaining disagreements were resolved by a discussion with a third and a fourth reviewer (Maurits P. A. van Meer, Matthijs P. Somford).
2.4 Source of funding
The authors received no financial support for this study.
3.1 Results of the search
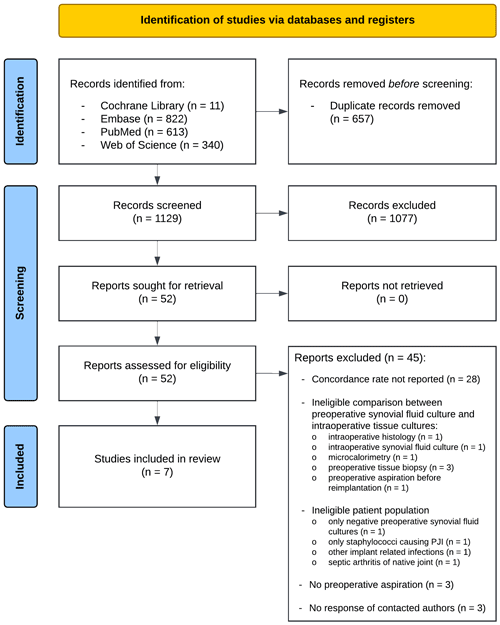
After removal of duplicates from the search results, 1129 titles and abstracts were screened. Of these, 52 were retrieved full-text to be assessed for eligibility. The majority of the ineligible studies were excluded because the concordance rate was not reported. Because no response was received upon a request to provide additional data from any of the latter papers' authors, these studies were also excluded . Ultimately, seven records, comprising 1677 patients, were eligible for inclusion (Fig. 1).
3.2 Study characteristics
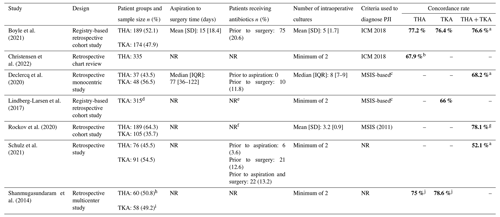
All included studies had a retrospective study design, of which five (71 %) explored patients undergoing revision surgery of both THA and TKA. One study reported on patients with THA revision surgery only and one on patients with TKA revision surgery only. There was a slight predominance of patients with a THA (53 %) compared to a TKA (47 %). Two studies reported the time interval between the preoperative aspiration and revision surgery, averaging 15 d (Boyle et al., 2021) and 77 d (Declercq et al., 2020), respectively. Two studies reported on the antibiotic administration prior to aspiration (0 %, Declercq et al., 2020, vs. 4 %, Schulz et al., 2021) and three (Boyle et al., 2021; Declercq et al., 2020; Schulz et al., 2021) reported that a percentage of patients received antibiotics preoperatively, which ranged between 12 % and 21 %.
Characteristics and a summary of results of the seven included are presented in Table 1. An overview of the concordance rates of the causative micro-organisms can be found in Table 2.
Table 1Characteristics of the seven included studies reporting concordance between preoperative synovial fluid cultures and intraoperative tissue cultures in patients with periprosthetic joint infection after total hip and knee arthroplasty.
Abbreviations: ICM: International Consensus Meeting; IQR: interquartile range; MSIS: Musculoskeletal Infection Society; NR: not reported; PJI: periprosthetic joint infection; SD: standard deviation; THA: total hip arthroplasty; TKA: total knee arthroplasty.
Legend: a Authors specifically did not include negative preoperative synovial fluid cultures. b Concordance rates in hip aspirations of a successful tap (n=215). c Authors did not specifically report the use of the MSIS criteria, but the criteria used to define PJI were similar to the MSIS criteria. d Preoperative knee joint aspiration was only performed in 157 of the 315 patients. e Authors only reported that “In both partial and two-stage revisions negative cultures were common … due to administration of antibiotics before surgery, of which [we have] no information in the current study.” f Authors only reported that “Cases were excluded if they […] had an antibiotic spacer in place at the time of aspiration.” g The concordance rate was only calculated for the patients diagnosed with PJI at the time of surgery (n=105). h Preoperative hip aspiration was only performed in 27 patients. i Preoperative knee aspiration was only performed in 31 patients. j Concordance rates have been calculated based on the discordance rates as reported by the authors.
Table 2Concordance rates of specific causative micro-organisms.
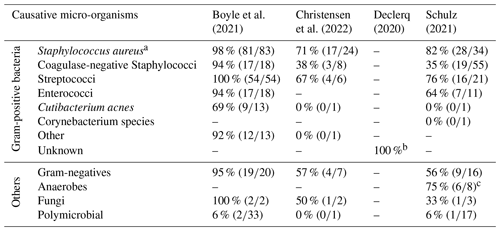
a This includes methicillin-resistant (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA). b Authors of this study did not explain how this percentage has been calculated, but only stated the following in their paper: “Our findings demonstrated that when translating the preoperative joint aspiration culture results to their corresponding Gram/fungi level, the preoperative joint aspiration culture yielding exclusively Gram-positive microorganisms, predicted Gram-positive causative pathogens in 100 % of cases.” c The authors stated that this group included the following: “Cutibacterium acnes (n=4), Peptostreptococcus micros (n=2), Bacteroides fragilis (n=1), Clostridium perfringens (n=1), Cutibacterium avidum (n=1), and Parvimonas micra (n=1).”
3.3 Risk of bias of the included studies
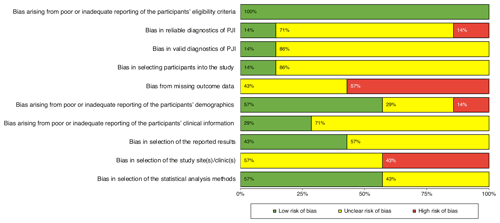
The summary of the risk of bias assessments for each of the seven included studies are shown in Fig. 2. Six studies (Boyle et al., 2021; Christensen et al., 2022; Lindberg-Larsen et al., 2017; Rockov et al., 2020; Schulz et al., 2021; Shanmugasundaram et al., 2014) were judged as having an unclear to high risk of bias and one (Declercq et al., 2020) as having a low risk of bias. Figure 3 shows that the items relating to reporting of the participants' eligibility criteria and the participants' demographics were judged as having a low risk of bias. None of the included studies were judged as having a low risk of bias from missing outcome data and in selection of the study site(s)/clinic(s). Also, the majority of included studies were judged as having an unclear to high risk of bias in the reliable or valid diagnosis of PJI, or because of poor or inadequate reporting of the participants' clinical information. There was also a high risk of bias in selecting participants into the study.
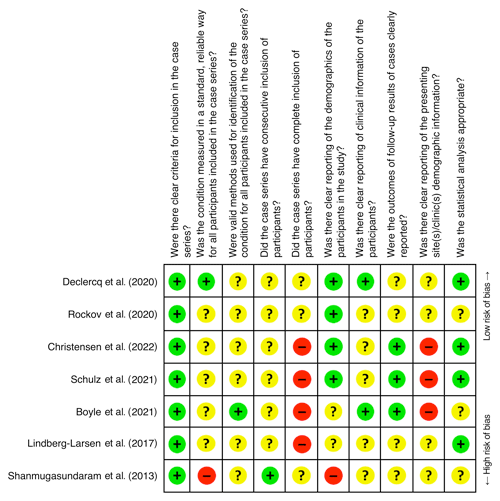
Figure 2Risk of bias summary: review authors' judgments about risk of bias items for each included study; – = high risk of bias; ? = unclear risk of bias; + = low risk of bias.
This review has identified and critically appraised the results of seven studies reporting on the concordance between preoperative synovial fluid cultures and intraoperative tissue cultures in over 1600 patients with PJI who were planned for knee or hip revision arthroplasty. Depending on how concordance was defined by the authors of the included studies, concordance rates varied between 45 % and 79 %. These rates were mainly produced by studies with an unclear to high risk of bias, which seriously hinders generalizability of the results. Only four studies (n=724) (Boyle et al., 2021; Christensen et al., 2022; Declercq et al., 2020; Schulz et al., 2021) analyzed the concordance between the types of PJI-causing micro-organisms, which was considered too limited to draw any useful conclusions.
All studies included in this review had a retrospective design. Because this design carries the risk of selection bias, evidence should ideally be based on level 1–2 studies. However, we found no such studies and as a consequence, it is difficult to generalize conclusions from this review to the overall population. Also, the included studies suffered from incomplete reporting of relevant medical data, and some studies did not report on the time interval between aspiration and surgery and whether patients received antibiotics before the aspiration or revision surgery. These several shortcomings highlight that there is currently no consensus about how the concordance between preoperative synovial fluid culture and intraoperative tissue cultures should be properly evaluated. Another clear example of this lack of consensus relates to how concordance rates were defined and subsequently calculated in the seven studies. In some, concordance was calculated based on the positive preoperative and positive intraoperative cultures only, whereas in others positive preoperative and positive intraoperative cultures as well as negative preoperative and negative intraoperative cultures were included in the calculations. In addition, Rockov et al. (2020) and Shanmugasundaram et al. (2014) did not define their concordance rates and this makes an overall comparison across all included studies challenging. We recommend that the concordance rate should include both the positive and negative concordant cultures. In the seven included studies, the highest concordance rate reported was 79 %. Boyle et al. (2021) specifically excluded cases with negative preoperative synovial fluid cultures and this may have led to overestimation of the concordance rate when compared to authors who included these.
In daily clinical practice, a concordance rate of 78 % between preoperative synovial fluid culture and intraoperative tissue cultures means that in about 1 in 3 to 4 cases, the results of the intraoperative tissue cultures do not match with the earlier results of the preoperative synovial fluid culture. So, when using the preoperative synovial culture as a single test to distinguish between septic or aseptic implant failure, 1 in 3 to 4 cases may be misdiagnosed and subsequently be under- or over-treated. To diagnose PJI, current guidelines recommend the use of preoperative synovial fluid aspiration culture combined with leukocyte count and percentage of polymorphonuclear neutrophils (Oliva et al., 2021; Signore et al., 2019; American Academy of Orthopaedic Surgeons (AAOS), 2019). The results of our review suggest that a clinician cannot confidently establish a postoperative treatment strategy based on the preoperative cultures alone. However, two studies suggest an exception when Gram-positive bacteria are found in the preoperative culture. This is illustrated by the reported concordance rates, showing that preoperative aspiration had a favorable concordance rate for Gram-positive bacteria – with the exception of Cutibacterium acnes – of 97 % (n=363) (Boyle et al., 2021) and 100 % (n=85) (Declercq et al., 2020) (Table 2). However, the concordance rates of the Gram-positive bacteria reported by two other studies (Christensen et al., 2022; Schulz et al., 2021) do not support this assumption. This needs to be confirmed using higher-quality studies, before the postoperative antibiotic regime could be narrowed when encountering Gram-positive bacteria in the preoperative aspiration culture.
Although the time between aspiration and surgery could potentially have an influence on concordance rates, exploring this association was not deemed worthwhile because only two studies (Boyle et al., 2021; Declercq et al., 2020) reported this time interval, which varied between a mean of 15 (n=363) and a median of 77 (n=85) d and concordance rates of 76.6 % and 63.5 %, respectively. Similarly, the use of antibiotics prior to aspiration and surgery was only reported in three studies (Boyle et al., 2021; Declercq et al., 2020; Schulz et al., 2021), leaving too little data for a proper analysis.
4.1 Risk of bias of the included studies
An important finding of this review was that the majority of the included studies were judged as having an unclear to high risk of bias. Studies were especially judged poorly on the items regarding the reporting of outcomes or follow-up results, study site selection, and the reliable and valid diagnosis of PJI. Especially the latter is disconcerting because in the past decade the use of MSIS and EBJIS diagnostic criteria have been endorsed to better assist clinicians in diagnosing PJI (Parvizi et al., 2018; McNally et al., 2021). In fact, multiple studies had to be excluded from this review because less than two intraoperative tissue cultures were obtained, even while the MSIS criteria published in 2011 already recommended that the diagnosis of PJI should only be established based on a minimum of two positive cultures (Parvizi et al., 2011). Overall, we only judged one (Declercq et al., 2020) of the seven studies as having a low risk of bias. This particular study reported a concordance rate of 68 %.
4.2 Limitations
This systematic review has two major limitations. First, we felt it was not appropriate to perform a meta-analysis because the majority of the included studies were of unclear to high-risk bias, which could have led to misleading results (Higgins et al., 2022). Second, we adapted JBI's critical appraisal checklist for case series to better suit the context of our review, but our adaptation was not assessed for inter-rater reliability nor validated beforehand. Regarding the latter, we purposely selected antibiotic use and the time between preoperative and intraoperative culture as two pieces of key clinical information that would need to be reported to properly judge their influence on the studies overall risk of bias. However, clinical information such as the patients' American Society of Anesthesiologists (ASA) classification or Charlson comorbidity index (CCI), body mass index (BMI), presence of inflammatory disease or diabetes, tobacco use, and the use of immunosuppressive medication may also be more or less important in this regard, so our choice of selecting and judging only two variables could be criticized.
4.3 Recommendations for clinical practice and future research
Based on the results of this systematic review, it is challenging to draw firm conclusions and make useful clinical practice recommendations for postoperative antibiotic therapy based on the preoperative synovial fluid culture. As long as there is no diagnostic test with high accuracy that can confirm the absence or presence of PJI pre- and intraoperatively, we recommend to adhere to the current practice guidelines. These guidelines recommend an empiric antibiotic regime as the standard for postoperative antibiotic treatment until the results of the intraoperative tissue cultures are known. Future prospective studies are needed, with attention to adequate participant selection by using the internationally recommended diagnostic criteria to establish PJI, detailed reporting of clinical information, relevant risk factors and outcomes, and a more detailed profile of the causative micro-organisms, such as the type and resistance profile, to determine a more precise estimate of the concordance rate between preoperative synovial fluid culture and intraoperative tissue cultures and the infection-causing micro-organisms in patients undergoing revision surgery of their THA or TKA.
The results of this systematic review show a wide range of reported concordance rates between preoperative synovial fluid culture and intraoperative tissue cultures and a high risk of bias in the studies reporting on these concordance rates. Higher-quality studies are warranted to obtain a better estimate of these concordance rates. Because the concordance between aspiration and tissue cultures has not yet been established, we do not recommend relying solely on the aspiration culture as a diagnostic tool for PJI. Instead, we recommend continuing the use of a system such as the EBJIS definition or MSIS criteria when diagnosing PJI.
The authors confirm that the data supporting the findings of this study are available within the article and its Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/jbji-7-259-2022-supplement.
TJAvS, LDdJ, and JHMG were responsible for the conceptualization. TJAvS and LDdJ were responsible for the investigation, formal analysis, methodology, and visualization. TJAvS was responsible for data curation, project administration, and the draft of the original paper. LDdJ, MPAvM, JHMG, and MPS were responsible for critical revision of the paper.
The contact author has declared that none of the authors has any competing interests.
An ethics statement is not applicable because this study is based exclusively on published literature.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We thank the Radboud University Library staff for their support during the literature search.
This paper was edited by Parham Sendi and reviewed by four anonymous referees.
Ahmad, S. S., Shaker, A., Saffarini, M., Chen, A. F., Hirschmann, M. T., and Kohl, S.: Accuracy of diagnostic tests for prosthetic joint infection: a systematic review, Knee Surg. Sports Traumatol. Arthrosc., 24, 3064–3074, https://doi.org/10.1007/S00167-016-4230-Y, 2016.
American Academy of Orthopaedic Surgeons: Diagnosis and Prevention of Periprosthetic Joint Infections Evidence-Based Clinical Practice Guideline, https://www.aaos.org/pjicpg (last access: 10 December 2022), 11 March 2019.
Atkins, B. L., Athanasou, N., Deeks, J., Crook, D. W. M., Simpson, H., Peto, T., Mclardy-Smith, P., Berendt, A. R., Benson, M., Carr, A., Collopy, D., Cooke, P., Kenwright, J., McClardy-Smith, P., Simpson, H., de Steiger, R., Gundle, R., Willett, K., Atkins, B., Berendt, A., Bowler, I., Conlon, C., Crook, D., Emptage, A., and Athanasou, N.: Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group, J. Clin. Microbiol., 36, 2932–2939, https://doi.org/10.1128/JCM.36.10.2932-2939.1998, 1998.
Boyle, K. K., Kapadia, M., Chiu, Y. F., Khilnani, T., Miller, A. O., Henry, M. W., Lyman, S., and Carli, A. V.: The James A. Rand Young Investigator's Award: Are Intraoperative Cultures Necessary If the Aspiration Culture Is Positive? A Concordance Study in Periprosthetic Joint Infection, J. Arthroplasty, 36, S4–S10, https://doi.org/10.1016/j.arth.2021.01.073, 2021.
Christensen, T. H., Ong, J., Lin, D., Aggarwal, V. K., Schwarzkopf, R., and Rozell, J. C.: How Does a “Dry Tap” Impact the Accuracy of Preoperative Aspiration Results in Predicting Chronic Periprosthetic Joint Infection?, J. Arthroplasty, 37, 925–929, https://doi.org/10.1016/j.arth.2022.01.066, 2022.
Declercq, P., Neyt, J., Depypere, M., Goris, S., van Wijngaerden, E., Verhaegen, J., Wauters, J., and Spriet, I.: Preoperative joint aspiration culture results and causative pathogens in total hip and knee prosthesis infections: mind the gap, Acta Clin. Belg., 75, 284–292, https://doi.org/10.1080/17843286.2019.1611718, 2020.
Fernández-Sampedro, M., Fariñas-Alvarez, C., Garces-Zarzalejo, C., Alonso-Aguirre, M. A., Salas-Venero, C., Martínez-Martínez, L., and Fariñas, M. C.: Accuracy of different diagnostic tests for early, delayed and late prosthetic joint infection, BMC Infect. Dis., 17, 592, https://doi.org/10.1186/s12879-017-2693-1, 2017.
Higgins, J., Thomas, J., Chandler, J., Cumpston M, Li, T., Page, M., and Welch, V.: Cochrane Handbook for Systematic Reviews of Interventions, version 6.3., edited by: Higgins, J., Thomas, J., Chandler, J., Cumpston M, Li, T., Page, M., and Welch, V., Cochrane, 2nd edn., John Wiley & Sons, Chichester (UK), 2022.
Holleyman, R. J., Deehan, D. J., Charlett, A., Gould, K., and Baker, P. N.: Does pre-operative sampling predict intra-operative cultures and antibiotic sensitivities in knee replacements revised for infection?: a study using the NJR dataset, Knee Surg. Sports Traumatol. Arthrosc., 24, 3056–3063, https://doi.org/10.1007/S00167-015-3841-Z, 2016.
Joanna Briggs Institute: Critical Appraisal Checklist for Case Series. https://jbi.global/critical-appraisal-tools (last access: 10 December 2022), 2017.
Kheir, M. M., Tan, T. L., Ackerman, C. T., Modi, R., Foltz, C., and Parvizi, J.: Culturing Periprosthetic Joint Infection: Number of Samples, Growth Duration, and Organisms, J. Arthroplasty, 33, 3531–3536.e1, https://doi.org/10.1016/J.ARTH.2018.06.018, 2018.
Li, H., Xu, C., Hao, L. B., Chai, W., Jun, F., and Chen, J.: The concordance between preoperative aspiration and intraoperative synovial fluid culture results: intraoperative synovial fluid re-cultures are necessary whether the preoperative aspiration culture is positive or not, BMC Infect. Dis., 21, 1018, https://doi.org/10.1186/s12879-021-06721-4, 2021.
Lindberg-Larsen, M., Pitter, F. T., Voldstedlund, M., Schrøder, H. M., and Bagger, J.: Microbiological diagnosis in revision of infected knee arthroplasties in Denmark, Infect. Dis. (Lond.), 49, 824–830, https://doi.org/10.1080/23744235.2017.1350878, 2017.
Lora-Tamayo, J., Murillo, O., Iribarren, J. A., Soriano, A., Sánchez-Somolinos, M., Baraia-Etxaburu, J. M., Rico, A., Palomino, J., Rodríguez-Pardo, D., Horcajada, J. P., Benito, N., Bahamonde, A., Granados, A., del Toro, M. D., Cobo, J., Riera, M., Ramos, A., Jover-Sáenz, A., and Ariza, J.: A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention, Clin. Infect. Dis., 56, 182–194, https://doi.org/10.1093/CID/CIS746, 2013.
Matter-Parrat, V., Ronde-Oustau, C., Boéri, C., Gaudias, J., and Jenny, J. Y.: Agreement between pre-operative and intra-operative bacteriological samples in 85 chronic peri-prosthetic infections, Orthop. Traumatol. Surg. Res., 103, 301–305, https://doi.org/10.1016/j.otsr.2016.11.022, 2017.
McInnes, M. D. F., Moher, D., Thombs, B. D., McGrath, T. A., Bossuyt, P. M., Clifford, T., Cohen, J. F., Deeks, J. J., Gatsonis, C., Hooft, L., Hunt, H. A., Hyde, C. J., Korevaar, D. A., Leeflang, M. M. G., Macaskill, P., Reitsma, J. B., Rodin, R., Rutjes, A. W. S., Salameh, J. P., Stevens, A., Takwoingi, Y., Tonelli, M., Weeks, L., Whiting, P., and Willis, B. H.: Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies The PRISMA-DTA Statement, JAMA, 319, 388–396, https://doi.org/10.1001/jama.2017.19163, 2018.
McNally, M., Sousa, R., Wouthuyzen-Bakker, M., Chen, A. F., Soriano, A., Vogely, H. C., Clauss, M., Higuera, C. A., and Trebše, R.: The EBJIS definition of periprosthetic joint infection, Bone Joint J., 103-B, 18–25, https://doi.org/10.1302/0301-620X.103B1.BJJ-2020-1381.R1, 2021.
Oliva, A., Miele, M. C., al Ismail, D., di Timoteo, F., de Angelis, M., Rosa, L., Cutone, A., Venditti, M., Mascellino, M. T., Valenti, P., and Mastroianni, C. M.: Challenges in the Microbiological Diagnosis of Implant-Associated Infections: A Summary of the Current Knowledge, Front. Microbiol., 12, 750460, https://doi.org/10.3389/fmicb.2021.750460, 2021.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., and Wilson, W. R.: Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America, Clin. Infect. Dis., 56, 1–10, https://doi.org/10.1093/CID/CIS966, 2013.
Ouzzani, M., Hammady, H., Fedorowicz, Z., and Elmagarmid, A.: Rayyan-a web and mobile app for systematic reviews, Syst. Rev., 5, 210, https://doi.org/10.1186/s13643-016-0384-4, 2016.
Pandey, R., Berendt, A. R., and Athanasou, N. A.: Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service, Arch. Orthop. Trauma Surg., 120, 570–574, https://doi.org/10.1007/S004020000174, 2000.
Parvizi, J., Zmistowski, B., Berbari, E. F., Bauer, T. W., Springer, B. D., della Valle, C. J., Garvin, K. L., Mont, M. A., Wongworawat, M. D., and Zalavras, C. G.: New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society, Clin. Orthop. Relat. Res., 469, 2992–2994, https://doi.org/10.1007/S11999-011-2102-9, 2011.
Parvizi, J., Tan, T. L., Goswami, K., Higuera, C., della Valle, C., Chen, A. F., and Shohat, N.: The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria, J. Arthroplasty, 33, 1309–1314, https://doi.org/10.1016/j.arth.2018.02.078, 2018.
Rockov, Z. A., Clarke, H. D., Grys, T. E., Chang, Y. H. H., and Schwartz, A. J.: Is There an Optimal Cutoff for Aspiration Fluid Volume in the Diagnosis of Periprosthetic Joint Infection?, J. Arthroplasty, 35, 2217–2222, https://doi.org/10.1016/j.arth.2020.03.011, 2020.
Schulz, P., Dlaska, C. E., Perka, C., Trampuz, A., and Renz, N.: Preoperative synovial fluid culture poorly predicts the pathogen causing periprosthetic joint infection, Infection, 49, 427–436, https://doi.org/10.1007/s15010-020-01540-2, 2021.
Shanmugasundaram, S., Ricciardi, B. F., Briggs, T. W. R., Sussmann, P. S., and Bostrom, M. P.: Evaluation and Management of Periprosthetic Joint Infection-an International, Multicenter Study, HSS J., 10, 36–44, https://doi.org/10.1007/s11420-013-9366-4, 2014.
Signore, A., Sconfienza, L. M., Borens, O., Glaudemans, A. W. J. M., Cassar-Pullicino, V., Trampuz, A., Winkler, H., Gheysens, O., Vanhoenacker, F. M. H. M., Petrosillo, N., and Jutte, P. C.: Consensus document for the diagnosis of prosthetic joint infections: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement), Eur. J. Nucl. Med. Mol. Imaging, 46, 971–988, https://doi.org/10.1007/s00259-019-4263-9, 2019.
Spangehl, M. J., Masri, B. A., O'Connell, J. X., and Duncan, C. P.: Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties, J. Bone Joint. Surg. Am., 81, 672–683, https://doi.org/10.2106/00004623-199905000-00008, 1999.
Tande, A. J. and Patel, R.: Prosthetic Joint Infection, Clin. Microbiol. Rev., 27, 302–345, https://doi.org/10.1128/CMR.00111-13, 2014.
Trampuz, A., Hanssen, A. D., Osmon, D. R., Mandrekar, J., Steckelberg, J. M., and Patel, R.: Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection, Am. J. Med., 117, 556–562, https://doi.org/10.1016/J.AMJMED.2004.06.022, 2004.
Vilchez, F., Martínez-Pastor, J. C., García-Ramiro, S., Bori, G., Maculé, F., Sierra, J., Font, L., Mensa, J., and Soriano, A.: Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement, Clin. Microbiol. Infect., 17, 439–444, https://doi.org/10.1111/J.1469-0691.2010.03244.X, 2011.
Wouthuyzen-Bakker, M., Sebillotte, M., Lomas, J., Taylor, A., Palomares, E. B., Murillo, O., Parvizi, J., Shohat, N., Reinoso, J. C., Sánchez, R. E., Fernandez-Sampedro, M., Senneville, E., Huotari, K., Barbero, J. M., Garcia-Cañete, J., Lora-Tamayo, J., Ferrari, M. C., Vaznaisiene, D., Yusuf, E., Aboltins, C., Trebse, R., Salles, M. J., Benito, N., Vila, A., Toro, M. D. del, Kramer, T. S., Petersdorf, S., Diaz-Brito, V., Tufan, Z. K., Sanchez, M., Arvieux, C., and Soriano, A.: Clinical outcome and risk factors for failure in late acute prosthetic joint infections treated with debridement and implant retention, J. Infect., 78, 40–47, https://doi.org/10.1016/j.jinf.2018.07.014, 2019.