the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Characteristics and outcomes of culture-negative prosthetic joint infections from the Prosthetic Joint Infection in Australia and New Zealand Observational (PIANO) cohort study
Sarah Browning
Laurens Manning
Sarah Metcalf
David L. Paterson
James O. Robinson
Benjamin Clark
Joshua S. Davis
Introduction: Culture-negative (CN) prosthetic joint infections (PJIs) account for approximately 10 % of all PJIs and present significant challenges for clinicians. We aimed to explore the significance of CN PJIs within a large prospective cohort study, comparing their characteristics and outcomes with culture-positive (CP) cases. Methods: The Prosthetic joint Infection in Australia and New Zealand Observational (PIANO) study is a prospective, multicentre observational cohort study that was conducted at 27 hospitals between 2014 and 2017. We compared baseline characteristics and outcomes of all patients with CN PJI from the PIANO cohort with those of CP cases. We report on PJI diagnostic criteria in the CN cohort and apply internationally recognized PJI diagnostic guidelines to determine optimal CN PJI detection methods. Results: Of the 650 patients with 24-month outcome data available, 55 (8.5 %) were CN and 595 were CP. Compared with the CP cohort, CN patients were more likely to be female (32 (58.2 %) vs. 245 (41.2 %); p = 0.016), involve the shoulder joint (5 (9.1 %) vs. 16 (2.7 %); p = 0.026), and have a lower mean C-reactive protein (142 mg L−1 vs. 187 mg L−1; p = 0.016). Overall, outcomes were superior in CN patients, with culture negativity an independent predictor of treatment success at 24 months (adjusted odds ratio, aOR, of 3.78 and 95 %CI of 1.65–8.67). Suboptimal diagnostic sampling was common in both cohorts, with CN PJI case detection enhanced using the Infectious Diseases Society of America PJI diagnostic guidelines. Conclusions: Current PJI diagnostic guidelines vary substantially in their ability to detect CN PJI, with comprehensive diagnostic sampling necessary to achieve diagnostic certainty. Definitive surgical management strategies should be determined by careful assessment of infection type, rather than by culture status alone.
- Article
(570 KB) - Full-text XML
-
Supplement
(637 KB) - BibTeX
- EndNote
Prosthetic joint infection (PJI) is a devastating complication of joint arthroplasty that is associated with significant patient morbidity and economic burden (Del Pozo and Patel, 2009; Moore et al., 2015). PJI complicates 1 %–2 % of primary arthroplasties (Del Pozo and Patel, 2009) and up to 8 % of cases following revision arthroplasty (Quinlan et al., 2020). Identification of pathogenic organisms from culture of synovial fluid and periprosthetic tissue is a cornerstone of PJI diagnosis and treatment decisions (Parvizi et al., 2018; McNally et al., 2021; Osmon et al., 2013). In approximately 10 % of all PJIs the diagnostic criteria indicating a PJI are met, but no causative pathogen is isolated (Reisener and Perka, 2018). In these situations, it is unclear if culture-negative (CN) PJI represents poor specificity of diagnostic criteria (i.e. not an infection), an infection caused by a fastidious non-culturable organism, or the absence of microbial growth as a consequence of antibiotic exposure prior to sampling. While previous retrospective analyses have been conducted, inconsistencies in inclusion criteria, classification of infection type, and treatment success measures limit the opportunity for direct comparisons and use in guiding clinical practice (Reisener and Perka, 2018; Peel et al., 2013).
We aimed to explore the significance of CN PJI within a large prospective cohort study and to compare their characteristics and outcomes with culture-positive (CP) cases.
2.1 Participants and setting
The Prosthetic joint Infection in Australia and New Zealand Observational (PIANO) study was a prospective, binational, multicentre observational cohort study that recruited at 27 hospitals between July 2014 and December 2017. A total of 783 patients with confirmed PJI were enrolled, comprising predominately knee (n=427), hip (n=323), and shoulder (n=25) joints. Detailed study methodology and baseline cohort characteristics (Manning et al., 2020) as well as outcomes after 24-months of follow-up have been described previously (Davis et al., 2022) and are summarized below.
Adult patients (> 18 years) with a newly diagnosed PJI were identified and enrolled following referral from an infectious diseases, microbiology, or orthopaedic team member. PJI was defined, using the modified Infectious Diseases Society of America (IDSA) PJI diagnostic guidelines, (Zimmerli, 2014) as the clinical suspicion of PJI in the presence of ongoing symptoms, including at least one of the following: (i) the presence of a sinus tract communicating with the prosthesis; (ii) increased leukocyte count or neutrophil percentage in preoperative synovial fluid aspirate (synovial fluid white blood cell count > 1700 cells µL−1 or neutrophil percentage > 65 %); (iii) visible pus around the prosthesis without an alternative explanation; (iv) acute inflammation reported on postoperative examination of periprosthetic tissue (≥ 5 neutrophils per high-power field); (v) ≥ 2 preoperative or intraoperative cultures (blood, synovial fluid, periprosthetic tissue, or sonication fluid) that yielded the same organism (indistinguishable based on common laboratory tests); or (vi) pure growth of Staphylococcus aureus, β-hemolytic streptococci, or pathogenic aerobic gram-negative rod from a single synovial fluid or intraoperative tissue/fluid specimen. Standard culture-based methods were used by all participating laboratories in processing blood cultures, synovial fluid, and periprosthetic tissue. The number and type of diagnostic samples were determined by local hospital policy and treating clinicians.
2.2 Definitions
Infections were classified according to the duration of symptoms and time from arthroplasty implantation, with the majority of patients classified as either “early postoperative” (diagnosis occurring ≤ 30 d after the original arthroplasty operation), “late acute” (diagnosis and onset of symptoms occurring > 30 d from implantation but with a total symptom duration of ≤ 7 d and no evidence of a sinus overlying the joint), or “chronic” (> 30 d from implantation and symptoms duration > 30 d at the time of diagnosis, or the presence of a sinus). Those with late-onset PJI, a duration of symptoms between 8 and 30 d, and without the presence of a sinus were considered to have late indeterminate infections, whereas the remainder were considered unclassifiable. CN PJIs were defined as those having met study inclusion criteria but with failure to isolate a causative pathogen from culture of synovial fluid or periprosthetic tissue specimens obtained at the time of, or prior to, the initial surgical intervention for suspected infection.
2.3 Data collection
Data were collected and recorded prospectively in a purpose-built web-based database at baseline, 3, 12, and 24 months post PJI diagnosis. The primary outcome measure was treatment success at 24 months follow-up, defined as being alive with no clinical or microbiological evidence of infection, no longer taking antibiotics for suppression or treatment of the PJI, and having the “key prosthesis” still in place. The key prosthesis was defined as follows: (i) the index prosthesis present at diagnosis for those whose main treatment strategy at day 90 was debridement and implant retention (DAIR); (ii) the destination prosthesis for those whose main treatment strategy at day 90 was two-stage revision, even if the second stage was completed after day 90; (iii) the destination prosthesis for those whose main treatment strategy at day 90 was single-stage revision; and (iv) the index prosthesis for those who managed with suppressive antibiotics with non-curative intent. All participants provided written informed consent. We performed a post hoc retrospective analysis of this prospectively collected data. Treatment was not dictated by authors but was purely observational in design.
2.4 Inclusion criteria
Of 783 patients enrolled, 653 had 24-month outcome data available and were screened for eligibility for inclusion in the analysis. Following detailed review, it was unclear in three cases whether modified IDSA PJI diagnostic criteria were met. These patients were subsequently excluded, with 650 patients remaining in the analysis.
Baseline demographic and clinical features were compared between CN and CP cohorts. A comparison of treatment success at 24 months post PJI diagnosis was performed and presented for the overall cohort as well as for infection type and 90 d surgical management strategy.
2.5 PJI diagnostic guidelines
In addition to the previously assessed modified IDSA diagnostic criteria, we applied the following diagnostic criteria to all CN cases: (i) 2013 International Consensus Meeting (ICM) (Parvizi et al., 2013); (ii) 2018 ICM (Parvizi et al., 2018), and (iii) 2021 European Bone and Joint Infection Society (EBJIS) (McNally et al., 2021). An erythrocyte sedimentation rate (ESR) was not available; hence, an elevated C-reactive protein (CRP) (> 10 mg L−1) was judged as having met the 2013 ICM diagnostic criteria for elevated inflammatory markers in this cohort.
2.6 Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics (version 26.0 Chicago, Illinois). Data were summarized using the mean (standard deviation) or median (interquartile range) for normally and non-normally distributed variables respectively. Categorical variables were compared using chi-squared tests or Fisher's exact tests as appropriate. Means of continuous variables were compared using an independent-samples t test where data were normally distributed, and medians were compared using a Mann–Whitney U test for nonparametric variables. Multivariate logistic regression using backward stepwise selection was used to determine independent predictors of success, with the 95 % confidence intervals presented. Two-tailed P values of < 0.05 were considered statistically significant.
3.1 Demographics
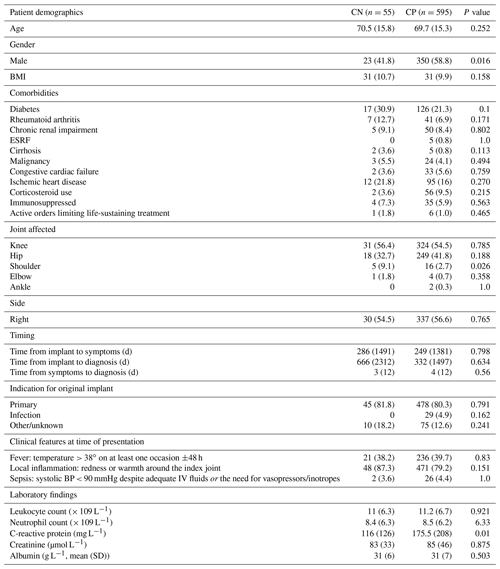
Of the 650 patients eligible for inclusion in this subgroup analysis, 55 (8.5 %) were classified as CN. The baseline characteristics of the CN patients did not differ from those of the CP patients (Table 1), apart from sex (CN more likely to be female), joint affected (CN more likely to be shoulder joint than CP), and mean baseline C-reactive protein (lower in CN cases).
Table 1Baseline characteristics of CN patients compared with CP patients.
The abbreviations used in the table are as follows: d – days, IV – intravenous, BMI – body mass index, ESRF – end-stage renal failure, and DAIR – debridement and implant retention. Data are no. (%) for categorical variables and median (interquartile range) for continuous variables, unless otherwise stated.
Late acute PJI was the most common CN infection type, present in 21 of 55 (38.2 %) cases. A total of 16 of 55 cases (29.1 %) were early postoperative, 12 of 55 cases (21.8 %) were chronic, and 6 of 55 cases (10.9 %) were not classifiable (1 late indeterminate and 5 late unclassifiable). No differences in infection type were observed between CN and CP groups. Two patients (3.6 %) died within the 24-month follow-up period.
No significant differences in diagnostic sampling were observed between CN and CP (Table 2). It is of note that only one laboratory routinely performed sonication, while 16S polymerase chain reaction (PCR) was not routinely performed or requested by any laboratory.
3.2 PJI diagnostic criteria
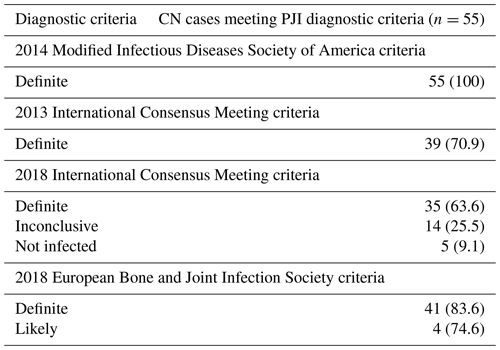
When compared to the 2014 modified IDSA criteria, the 2013 ICM (70.9 %), 2018 ICM (63.6 %), and 2021 EBJIS (definite and likely) (80 %) criteria were less likely to diagnose PJI in this cohort (Table 3). Amongst the 10 CN patients who failed to meet the 2021 EBJIS diagnostic criteria, suboptimal diagnostic sampling and visible purulence around the prosthesis were found to be uniformly present (see Table S1 in the Supplement). Following detailed review, only 1 of these 10 cases (patient no. 6, Table S1) was deemed unlikely to have a true PJI, having failed to demonstrate definitive histopathological or microbiological evidence of infection despite an extensive diagnostic workup having been performed.
3.3 Surgical management and 24-month treatment success
Within the CN cohort, DAIR was the most common surgical treatment strategy (30 of 55 cases, 54.6 %), followed by two-stage revision (13 of 55 cases, 23.6 %) (Table 4).
Table 4Infection type, index surgery, and treatment success in culture-negative and culture-positive prosthetic joint infections.
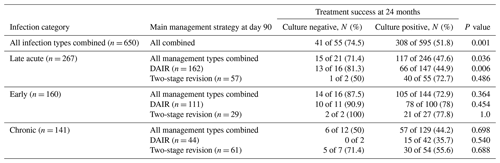
Despite this, culture negativity was associated with an increased likelihood of treatment success overall (41 of 55 cases, 74.5 %, vs. 308 of 595 cases, 51.8 %; p = 0.001), and it was an independent predictor of treatment success at 24 months (adjusted odds ratio, aOR, of 3.78 and 95 %CI of 1.65–8.67) (Table 5).
Table 5Independent predictors of treatment success at 24 months (n = 650).
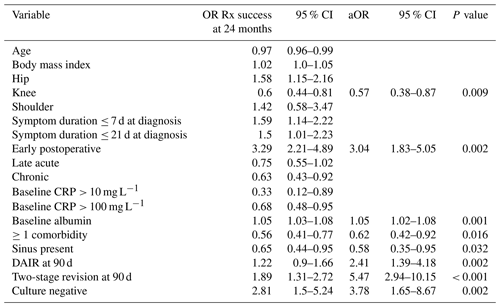
“OR Rx success” refers to the odds ratio of treatment success.
By infection type, improved outcomes were demonstrated only in late acute CN PJIs and was most pronounced in those managed with DAIR (13 of 17 cases, 81.3 %, vs. 66 of 147 cases, 44.9 %; aOR of 5.3 and 95 %CI of 1.5–19.5) (Tables 4, S2).
This multicentre retrospective analysis offers important insights into the presenting features, demographics, and expected treatment outcomes in CN PJI. In contrast to previous reports (Parikh and Antony, 2015), our findings do not support the notion that atypical pathogens play a significant role in CN PJI. Instead, striking similarities between CN and CP patients mean that risk factors, such antibiotic exposure, suboptimal sampling practices, and delays in laboratory processing, are more likely to be implicated (Kang et al., 2018).
Of those eligible for study inclusion, 8.5 % were classified as being CN, which is lower than the reported pooled incidence rate of 11 % (Reisener and Perka, 2018). This may relate to differences in diagnostic approaches or laboratory processing methods across geographical locations and health networks. It is of note that sonication was only routinely used at one of the participating laboratories.
Overall, baseline demographics were similar between the CN and CP cohorts. However, it is interesting to note the higher proportion of shoulder infections within the CN cohort, which is likely explained by difficulties encountered in the isolation of Cutibacterium acnes using typical culture methods, with an extended incubation time of up to 14 d sometimes required (Foster et al., 2021). While standard culture techniques were utilized by all participating laboratories, the duration of extended culture was not standardized and may have resulted in the reduced sensitivity of pathogen detection. In contrast to previously studied CN cohorts (Choi et al., 2013; Kim et al., 2015), late acute PJI was found to be the predominant infection type; while heterogenous infection type definitions may have contributed to this (Tsukayama et al., 1996), crystal arthropathy as a mimicker of bacterial PJI may provide an alternate explanation for this finding (Khalfaoui and Yassa, 2015).
The EBJIS criteria were not met for 10 (18.2 %) of the CN cohort. This could either mean that the 2021 EBJIS criteria are less sensitive than the 2014 modified IDSA criteria or that they are more specific. As there is no gold standard test for PJI in the absence of culture positivity, it is not possible to definitively say which of these is the true explanation.
Both the number and type of periprosthetic specimens submitted for bacterial culture are known to impact upon the sensitivity and specificity of pathogen detection in PJIs (Peel et al., 2017). While the mean number of specimens collected was less than guideline-based recommendations (Osmon et al., 2013; Peel et al., 2017), this did not appear to contribute to a reduction in identifiable causative pathogens, with no significant differences in diagnostic sampling observed between the two groups. Suboptimal diagnostic sampling methods did appear to impact on PJI diagnostic certainty, however, with a notable absence of histopathological examination and synovial fluid analysis in the majority of the 10 cases that failed to meet the 2021 EBJIS PJI criteria (Table S1). Instead, visible purulence around the prosthesis was the sole definitive diagnostic criterion present in all 10 cases. While the value of visible purulence has been questioned due to its subjective nature and poor specificity (Alijanipour et al., 2015), our findings highlight the potential value of this diagnostic criterion when applied in a real-life setting, where suboptimal diagnostic sampling is frequently encountered.
In accordance with existing literature about CN PJI (Kang et al., 2018; Choi et al., 2013; Paz et al., 2021), we demonstrate improved overall outcomes when compared with CP cases, despite no observed difference in the incidence of fever or sepsis between cohorts (Paz et al., 2021). When compared with CP patients, CN late acute infections managed with DAIR were 5.3 times more likely to experience treatment success (95 %CI of 1.5–19.5). This finding has not been demonstrated in other CN cohorts (Kim et al., 2015; Choi et al., 2013) but may be explained by the factors outlined in the following. Firstly, pre-administration of antibiotics is a known predictor of CN PJI (Berbari et al., 2007; Malekzadeh et al., 2010); hence, in late acute presentations, CN PJI may represent a cohort in which the causative pathogen is more readily eradicated or suppressed. A lack of biofilm formation and increased pathogen susceptibility does not provide a complete explanation for these findings, however, given similar outcomes observed in early postoperative CN and CP cohorts. Instead, this finding further supports the concept that late acute PJI represents a heterogenous cohort, where a subset of CN late acute infections may represent chronic bacterial infections with short overt symptom durations (Davis et al., 2022). Additionally, crystal arthropathies due to monosodium urate and calcium pyrophosphate dihydrate deposition do not form part of routine PJI investigation and may account for a proportion of late acute presentations and improved outcomes. While further inaccuracies in the diagnosis of CN PJI could also explain these findings, detailed examination of the presenting features and diagnostic criterion of this cohort (see Table S2) does not support this.
Our study does have some limitations. For example, antibiotic exposure prior to diagnostic sampling was not measured in either cohort, which may have contributed to culture negativity in the absence of other major demographic differences. While data were collected prospectively, the retrospective nature of this analysis and missing data mean that definitive conclusions are unable to be drawn with regards to the sensitivity and utility of current diagnostic criteria in the setting of CN PJI.
Despite improved outcomes, CN PJI continues to represent a significant management challenge for surgeons and physicians alike. As such, clarity with regards to optimal surgical management strategies is needed. While it has been previously recommended that two-stage revisions be considered as the optimal definitive surgical management strategy in all CN PJIs (Ibrahim et al., 2018), our findings instead emphasize the importance of precise infection-type classifications in allowing for a more nuanced approached to CN PJI diagnosis and management. This important interplay has been described previously (Davis et al., 2022) and highlights a crucial need to base definitive surgical management decisions on PJI infection type using standardized classification methods, regardless of culture status.
The use of a standardized yet comprehensive approach to PJI diagnostic sampling is reliant upon the collaborative efforts of infectious diseases physicians, pathologists, and orthopaedic surgeons, and it should be guided by available evidence to optimize the sensitivity and specificity of PJI detection. In high-volume centres, preprepared sterile sampling kits have the potential to optimize sampling technique and uniformity (Larsen et al., 2014), while laboratory-based protocols addressing PJI specimen processing and multidisciplinary case-based discussions should be implemented to foster a culture of best practice and improve PJI outcomes.
In conclusion, CN PJIs are almost indistinguishable from a broader CP PJI cohort while possessing an overall improved prognosis. When applied in a real-life setting, current PJI diagnostic guidelines vary substantially in their ability to detect CN PJI; this highlights the importance of comprehensive diagnostic sampling, including non-culture-based methods, at the time of any revision arthroplasty or DAIR procedure. Like CP PJI, definitive surgical management strategies should be determined by careful assessment of infection type, rather than by culture status alone.
The PIANO dataset is not yet publicly available, as preplanned analyses by the PIANO study group are ongoing. In the meantime, a de-identified version of the dataset can be accessed by submitting a research proposal to the PIANO study management committee (who are the data custodians) for approval. Proposals can be addressed to Joshua Davis (joshua.davis@health.nsw.gov.au).
The supplement related to this article is available online at: https://doi.org/10.5194/jbji-7-203-2022-supplement.
LM, SM, DLP, JOR, BC, and JSD were responsible for conceptualization and investigation. JSD provided supervision for SB with respect to the methodology, formal analysis, and composition of the manuscript. LM, SM, DLP, JOR, BC, and JSD assisted in the reviewing and editing of the manuscript.
The contact author has declared that none of the authors has any competing interests.
Ethical approvals were obtained from each participating site, and the PIANO study was prospectively registered (ANZCTR12615001357549). All participants provided written informed consent.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We wish to acknowledge the contributions of the PIANO investigators: Stephen Graves, Eugene Athan, Chris Luey, Paul Huggan, Kate Grimwade, Kerry Read, Piers Yates, Renjy Nelson, Marjoree Sehu, Adrienne Torda, Alistair Reid, Craig Aboltins, Peter Leung, Thi Aung, Roy Chean, Darcie Cooper, Babak Rad, Archana Sud, Kellie Schneider, Vana Nagendra, Stephen McBride, Mark Loewenthal, David Looke, Christopher Lemoh, David Campbell, Lucian Bogdan Solomon, Nora Mutalima, and Tony Allworth.
This paper was edited by Parham Sendi and reviewed by three anonymous referees.
Alijanipour, P., Adeli, B., Hansen, E. N., Chen, A. F., and Parvizi, J.: Intraoperative purulence is not reliable for diagnosing periprosthetic joint infection, J Arthroplasty, 30, 1403–1406, 2015.
Berbari, E. F., Marculescu, C., Sia, I., Lahr, B. D., Hanssen, A. D., Steckelberg, J. M., Gullerud, R., and Osmon, D. R.: Culture-Negative Prosthetic Joint Infection, Clin. Infect. Dis., 45, 1113–1119, https://doi.org/10.1086/522184, 2007.
Choi, H.-R., Kwon, Y.-M., Freiberg, A. A., Nelson, S. B., and Malchau, H.: Periprosthetic joint infection with negative culture results: clinical characteristics and treatment outcome, J. Arthroplasty, 28, 899–903, 2013.
Davis, J. S., Metcalf, S., Clark, B., Robinson, J. O., Huggan, P., Luey, C., McBride, S., Aboltins, C., Nelson, R., Campbell, D., Solomon, L. B., Schneider, K., Loewenthal, M. R., Yates, P., Athan, E., Cooper, D., Rad, B., Allworth, T., Reid, A., Read, K., Leung, P., Sud, A., Nagendra, V., Chean, R., Lemoh, C., Mutalima, N., Tran, T., Grimwade, K., Sehu, M., Looke, D., Torda, A., Aung, T., Graves, S., Paterson, D. L., and Manning, L.: Predictors of Treatment Success After Periprosthetic Joint Infection: 24-Month Follow up From a Multicenter Prospective Observational Cohort Study of 653 Patients, Open Forum Infectious Diseases, 9, ofac048, https://doi.org/10.1093/ofid/ofac048, 2022.
Del Pozo, J. L. and Patel, R.: Infection Associated with Prosthetic Joints, New Engl. J. Med., 361, 787–794, https://doi.org/10.1056/NEJMcp0905029, 2009.
Foster, A. L., Cutbush, K., Ezure, Y., Schuetz, M. A., Crawford, R., and Paterson, D. L.: Cutibacterium acnes in shoulder surgery: a scoping review of strategies for prevention, diagnosis, and treatment, J. Shoulder Elb. Surg., 30, 1410–1422, https://doi.org/10.1016/j.jse.2020.11.011, 2021.
Ibrahim, M., Twaij, H., and Haddad, F.: Two-stage revision for the culture-negative infected total hip arthroplasty: A comparative study, Bone Joint J., 100, 3–8, 2018.
Kang, J.-S., Shin, E.-H., Roh, T.-H., Na, Y., Moon, K. H., and Park, J.-H.: Long-term clinical outcome of two-stage revision surgery for infected hip arthroplasty using cement spacer: Culture negative versus culture positive, J. Orthop. Surg.-Hong K., 26, 2309499017754095, https://doi.org/10.1177/2309499017754095, 2018.
Khalfaoui, M. Y. and Yassa, R.: Crystal arthropathy following knee arthroplasty: A review of the literature, Int. J. Orthop., 2, 411–417, 2015.
Kim, Y.-H., Kulkarni, S. S., Park, J.-W., Kim, J.-S., Oh, H.-K., and Rastogi, D.: Comparison of infection control rates and clinical outcomes in culture-positive and culture-negative infected total-knee arthroplasty, J. Orthop., 12, S37–S43, https://doi.org/10.1016/j.jor.2015.01.020, 2015.
Larsen, L. H., Xu, Y., Simonsen, O., Pedersen, C., Schønheyder, H. C., Thomsen, T. R., and Group, P. S.: “All in a box” a concept for optimizing microbiological diagnostic sampling in prosthetic joint infections, BMC Research Notes, 7, p. 418, https://doi.org/10.1186/1756-0500-7-418, 2014.
Malekzadeh, D., Osmon, D. R., Lahr, B. D., Hanssen, A. D., and Berbari, E. F.: Prior Use of Antimicrobial Therapy is a Risk Factor for Culture-negative Prosthetic Joint Infection, Clin. Orthop. Relat. R., 468, 2039–2045, https://doi.org/10.1007/s11999-010-1338-0, 2010.
Manning, L., Metcalf, S., Clark, B., Robinson, J. O., Huggan, P., Luey, C., McBride, S., Aboltins, C., Nelson, R., Campbell, D., Solomon, L. B., Schneider, K., Loewenthal, M., Yates, P., Athan, E., Cooper, D., Rad, B., Allworth, T., Reid, A., Read, K., Leung, P., Sud, A., Nagendra, V., Chean, R., Lemoh, C., Mutalima, N., Grimwade, K., Sehu, M., Torda, A., Aung, T., Graves, S., Paterson, D., and Davis, J.: Clinical Characteristics, Etiology, and Initial Management Strategy of Newly Diagnosed Periprosthetic Joint Infection: A Multicenter, Prospective Observational Cohort Study of 783 Patients, Open Forum Infectious Diseases, 7, ofaa068, https://doi.org/10.1093/OFID/OFAA068, 2020.
McNally, M., Sousa, R., Wouthuyzen-Bakker, M., Chen, A. F., Soriano, A., Vogely, H. C., Clauss, M., Higuera, C. A., and Trebše, R.: The EBJIS definition of periprosthetic joint infection, Bone Joint. J., 103-b, 18–25, https://doi.org/10.1302/0301-620x.103b1.Bjj-2020-1381.R1, 2021.
Moore, A. J., Blom, A. W., Whitehouse, M. R., and Gooberman-Hill, R.: Deep prosthetic joint infection: a qualitative study of the impact on patients and their experiences of revision surgery, BMJ Open, 5, e009495, https://doi.org/10.1136/bmjopen-2015-009495, 2015.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., and Wilson, W. R.: Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America, Clin. Infect. Dis., 56, 1–10, https://doi.org/10.1093/cid/cis803, 2013.
Parikh, M. S. and Antony, S.: A comprehensive review of the diagnosis and management of prosthetic joint infections in the absence of positive cultures, J. Infect. Public Heal., 9, 545–556, https://doi.org/10.1016/j.jiph.2015.12.001, 2015.
Parvizi, J., Gehrke, T., and Chen, A.: Proceedings of the international consensus on periprosthetic joint infection, Bone Joint J., 95, 1450–1452, 2013.
Parvizi, J., Tan, T. L., Goswami, K., Higuera, C., Della Valle, C., Chen, A. F., and Shohat, N.: The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria, J. Arthroplasty, 33, 1309-1314.e1302, https://doi.org/10.1016/j.arth.2018.02.078, 2018.
Paz, Z., Zhu, C., Lieber, S. B., Fowler, M. L., and Shmerling, R. H.: Presentation and Outcomes of Peri-Prosthetic Joint Infection: A Comparison of Culture-Positive and Culture-Negative Disease, Surg. Infect., 22, 828–835, https://doi.org/10.1089/sur.2020.302, 2021.
Peel, T. N., Dowsey, M. M., Aboltins, C. A., Daffy, J. R., Stanley, P. A., Buising, K. L., and Choong, P. F.: Culture negative prosthetic joint infection – a description of current treatment and outcomes, Clin. Microbiol., 2, 1–5, 2013.
Peel, T. N., Spelman, T., Dylla, B. L., Hughes, J. G., Greenwood-Quaintance, K. E., Cheng, A. C., Mandrekar, J. N., and Patel, R.: Optimal periprosthetic tissue specimen number for diagnosis of prosthetic joint infection, J. Clin. Microbiol., 55, 234–243, 2017.
Quinlan, N. D., Werner, B. C., Brown, T. E., and Browne, J. A.: Risk of Prosthetic Joint Infection Increases Following Early Aseptic Revision Surgery of Total Hip and Knee Arthroplasty, J. Arthroplasty, 35, 3661–3667, https://doi.org/10.1016/j.arth.2020.06.089, 2020.
Reisener, M. and Perka, C.: Do Culture-Negative Periprosthetic Joint Infections Have a Worse Outcome Than Culture-Positive Periprosthetic Joint Infections? A Systematic Review and Meta-Analysis, BioMed Res. Int., 2018, 6278012, https://doi.org/10.1155/2018/6278012, 2018.
Tsukayama, D. T., Estrada, R., and Gustilo, R. B.: Infection after Total Hip Arthroplasty. A Study of the Treatment of One Hundred and Six Infections, J. Bone Joint Surg. Am., 78, 512–523, https://doi.org/10.2106/00004623-199604000-00005, 1996.
Zimmerli, W.: Clinical presentation and treatment of orthopaedic implant-associated infection, J. Intern. Med., 276, 111–119, https://doi.org/10.1111/joim.12233, 2014.