the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Diagnosis and management of fracture-related infections in a low-income country: a prospective study comparing current practice to international consensus guidelines
Elizabeth K. Tissingh
Cilia Ngang
Olivier Kennedy Muluem
Jasmine Sibatcheu Simo
Richard Douvamai
Jean Bahebeck
Olivier Cornu
Martin McNally
Data on the implementation of international consensus guidelines for fracture-related infection (FRI) in low- and middle-income countries (LMICs) are scarce. This study assessed whether FRI diagnosis and management in an LMIC align with these recommendations. Methods: We conducted a prospective multicenter study across four tertiary hospitals in Yaoundé, Cameroon (September 2022–July 2025). All consecutive patients with a working FRI diagnosis were included. Confirmatory/suggestive diagnostic criteria and treatment strategies were assessed against consensus guidelines. Results: A total of 169 patients were included (mean age 39.4 ± 15.4 years; 72.7 % male). In 34.3 % of cases, FRI occurred without prior surgery, limiting applicability of the Willenegger and Roth classification. Clinical confirmatory criteria were present in 97 % of cases. Microbiological standards were seldom achieved: none fulfilled sampling quantity, and only 46.6 % met sampling method recommendations. A microbiological confirmatory criterion was documented in 36 patients (21.3 %); histopathology was rarely performed (1.2 %), and nuclear imaging was not used. Most patients (81.1 %) were on antibiotics before admission or surgery. The most common treatment strategies were suppressive antibiotic therapy (44.4 %); one-stage (11.2 %) or two-stage (10.7 %) debridement, antibiotics, and implant exchange (DAIEX); and debridement, antibiotics, and implant retention (DAIR; 9.5 %). Overall, 62.7 % of treatments deviated from consensus guidelines. Conclusion: Nearly two-thirds of FRIs in this LMIC setting were managed outside international consensus guidelines. While the consensus definition is applicable, diagnostic capacity remains limited and microbiological standards are often impractical. Context-adapted, evidence-based guidelines are urgently needed to improve outcomes where the burden is highest.
- Article
(918 KB) - Full-text XML
- BibTeX
- EndNote
Fracture-related infection (FRI) is highly prevalent in low- and middle-income countries (LMICs), particularly in sub-Saharan Africa, where its incidence can reach up to 52 % following open fractures (Fonkoue et al., 2023b; Kouassi et al., 2019; Whiting et al., 2019) and 5 %–9 % after surgery of closed fractures (Fonkoué et al., 2024b; Saris et al., 2006). Although it receives limited global attention, most FRI cases occur and are managed in LMICs (Tissingh et al., 2022). Various constraints, such as delayed access to care, limited diagnostic resources, and inadequate infrastructure, render their management particularly challenging (Tissingh et al., 2022; Metsemakers et al., 2023). As a result, LMICs are believed to bear the highest burden of FRI worldwide (Metsemakers et al., 2023; Tissingh et al., 2022).
International expert groups – including the Fracture-Related Infection (FRI) Consensus Group and the International Consensus Meeting (ICM) Orthopaedic Trauma Work Group – have issued standardized definitions, diagnostic criteria, and treatment guidelines (Govaert et al., 2020; Metsemakers et al., 2018; Obremskey et al., 2020). These guidelines, primarily developed by experts from high-income countries (HICs), rely on several key elements (Metsemakers et al., 2020; Rupp et al., 2023; Bezstarosti et al., 2019; Depypere et al., 2020a; Prada et al., 2022; Vicenti et al., 2024; Foster et al., 2020): a multidisciplinary team (MDT) approach, host optimization, surgery, and combined systemic and local antimicrobial therapy. The main surgical concepts are (1) debridement, antibiotics, and implant retention (DAIR) in the case of early infection with a good fracture reduction and stable construct and (2) debridement, antibiotics, and implant exchange (DAIEX), performed in one or multiple stages, or implant removal if the fracture has consolidated (Metsemakers et al., 2020; Marais et al., 2024a).
In addition to surgery, the second cornerstone of FRI treatment is antimicrobial therapy. Antibiotics should be withheld until deep tissue sampling, except in sepsis. Guidelines for microbiological analysis recommend five deep tissue samples and collaboration with infectious disease specialists for antimicrobial stewardship (Dudareva et al., 2021). Local antibiotics delivered via carriers are strongly encouraged (Metsemakers et al., 2020; Unsworth et al., 2024; Sliepen et al., 2022). Suppressive therapy is reserved for difficult-to-treat cases or when surgery is not feasible, with antibiotics continued until fracture union (Metsemakers et al., 2020; Tsang et al., 2024).
These recommendations have demonstrated improved outcomes in HICs (Rupp et al., 2023; McNally et al., 2022). However, their implementation in LMICs is hampered by financial barriers, lack of insurance, scarce diagnostic tools, uneven surgical expertise, and limited access to updated literature (Tissingh et al., 2022; Tsang et al., 2024; Metsemakers et al., 2023). Moreover, guidelines were developed without LMIC representation, raising concerns about applicability (Tissingh et al., 2022). To date, there is a critical lack of data on how FRI is diagnosed and managed in LMICs (Metsemakers et al., 2023; Tissingh et al., 2022; Tsang et al., 2024), although a context-adapted guideline has recently become available (ABJIN, 2025). Therefore, this study assessed diagnostic and treatment practices for FRI across tertiary hospitals in Cameroon, evaluated adherence to international guidelines, and identified areas for intervention to improve care in resource-limited contexts.
2.1 Ethics approval
The Institutional Review Board of the University of Yaoundé I, Cameroon, approved the study protocol (Ethical clearance No 0953/UY1/FMSB). Patients or their legal guardians provided informed consent for participation.
2.2 Study design
We conducted a prospective, consecutive, observational cohort study including all patients diagnosed and treated for FRI in four tertiary hospitals in Yaoundé, Cameroon, between September 2022 and July 2025. All patients, of any age, in whom limb FRI was suspected or confirmed and documented as such in the medical records by the treating clinical team, were included. For each patient with a working diagnosis of FRI, investigators assessed the presence of confirmatory and suggestive diagnostic criteria according to the 2018 International FRI Consensus Group Definition (Metsemakers et al., 2018; Govaert et al., 2020).
2.3 Data collection
Data included demographics, comorbidities, index fracture details, clinical presentation, and laboratory and radiologic findings. All confirmatory and suggestive criteria were assessed, and FRIs were classified according to Willenegger and Roth (Willenegger and Roth, 1986). For each case, management was evaluated across multiple dimensions. An MDT approach was considered present if the case was discussed in a meeting with ≥2 specialists (orthopedic surgeon, plastic surgeon, microbiologist, infectious disease specialist, endocrinologist, or radiologist). Patients were “optimized” if comorbidities (e.g., diabetes, obesity, malnutrition, smoking, vascular disease, HIV) had been addressed pre-operatively.
Surgical data included delay from injury to FRI diagnosis, procedure type (DAIR, DAIEX, or other), number of surgeries, dead space management, local antibiotics, fixation, and soft tissue coverage. Microbiological assessment documented sampling method, number of samples, and pathogens. Antimicrobial therapy was evaluated for timing, duration, and adequacy.
Two investigators assessed adherence to core consensus principles using the algorithm by Depypere et al. (2020b). DAIR was appropriate if the implant was stable, symptoms were <10 weeks, host status was favorable, soft tissue was viable with sufficient coverage, and there was no intramedullary nail (Baertl et al., 2024; Vicenti et al., 2024). Suppressive therapy was deemed appropriate for type C hosts (Cierny and Mader) or during active fracture healing with callus formation (Tsang et al., 2024; Depypere et al., 2020b; Metsemakers et al., 2020). Debridement with implant removal was considered suitable after fracture union. In all other scenarios, implant exchange (DAIEX) in one or two stages was considered the appropriate strategy. Antibiotic therapy was considered appropriate if culture-specific antibiotics were administered for a total of 6 weeks in cases without an implant or following implant removal and for 12 weeks in cases of implant retention or exchange (Depypere et al., 2020a, b, Asim et al., 2024).
2.4 Statistical analysis
Statistical analyses were performed using SPSS software, version 26.0 (IBM Corp., Chicago, IL, USA). Categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as means ± standard deviation (SD). Associations between timing of infection onset and treatment approach, along with adherence to guidelines and clinical outcome, were tested with chi-squared analysis. Statistical significance was set at p<0.05.
3.1 Patient demographics and index fracture details
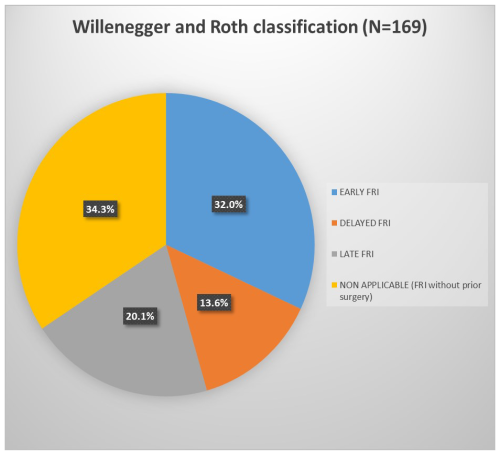
During the study period, 169 patients were diagnosed and treated for FRI. Baseline characteristics are summarized in Table 1. Most patients were young males from low–middle socioeconomic backgrounds and otherwise healthy (ASA I). FRIs mainly followed open fractures (63 %), involving predominantly the tibia and femur. Index fractures were most often fixed with external fixation or plates, but over one-third were managed non-surgically. The mean interval from injury to FRI diagnosis was 50.0 ± 106.7 d. According to Willenegger and Roth, FRIs were early in 54 cases (32.0 %), delayed in 23 cases (13.6 %), and late in 34 cases (20.1 %) (Fig. 1). The 58 patients without prior surgery – 51 open fractures and 7 treated by bonesetters – could not be classified with this system, as it is based on the interval between index surgery and onset of infection. This could not be applied as no surgical time point was available.
3.2 Diagnostic criteria
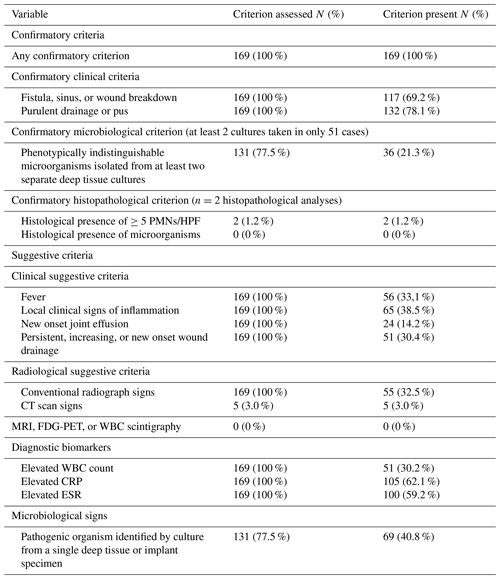
The prevalence of confirmatory and suggestive diagnostic criteria is shown in Table 2. All patients had at least one confirmatory criterion, with 164 (97 %) displaying a clinical confirmatory sign. Microbiological analysis was performed in 131 (77.5 %) cases, with confirmatory criteria met in only 36 (21.3 %) patients, largely due to insufficient sampling (≥2 samples in only 39.0 % of patients). Histopathology was done in only 2 cases (1.2 %), both positive. Among suggestive criteria, local signs of inflammation (N = 65, 38.5 %), fever (N = 56, 33.1 %), and wound drainage (N = 51, 30.4 %) were most common. Elevated white blood cell (WBC) count and CRP were observed in 51 (30.2 %) and 105 (62.1 %) patients, respectively. Plain radiographic signs were present in 55 patients (32.5 %). Only 5 patients (3.0 %) underwent CT scans. No MRI, 18FDG-PET, or scintigraphy was performed. Pathogens were isolated from a single culture sample in 69 cases (40.8 %).
3.3 Microbiological findings
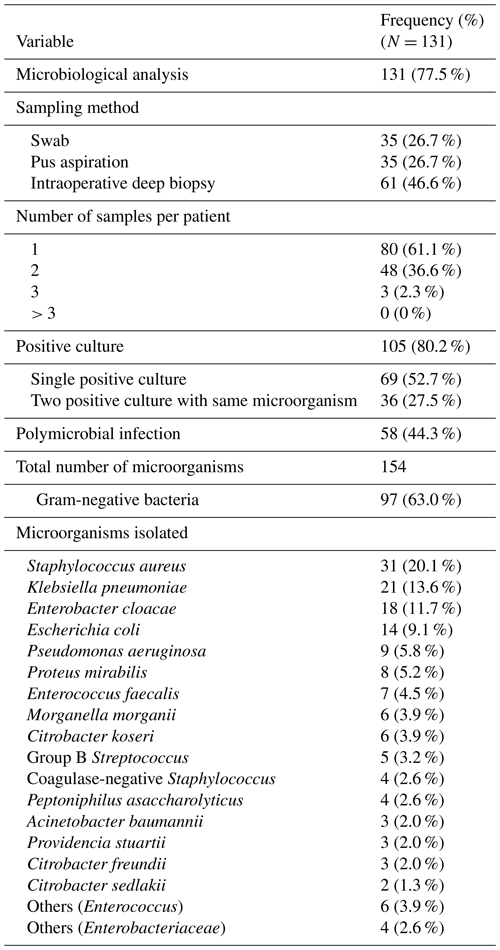
Of 131 patients (77.5 %) who underwent microbiological analysis, intraoperative biopsies were obtained in 61 (46.6 %) cases. The mean number of samples was 1.4 ± 0.5 (range 1–3); none had ≥5 as recommended. Cultures were positive in 105 patients (80.2 %). Infections were polymicrobial in 58 cases (44.3 %), and Gram-negative bacteria were predominant (N = 97, 63 %).
3.4 Management
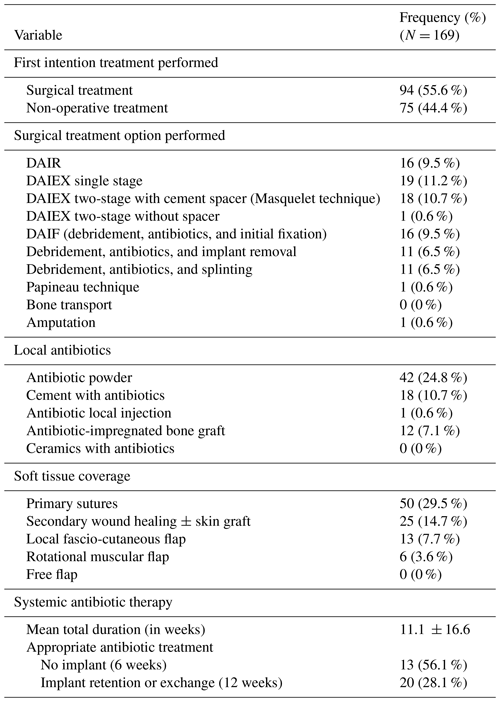
No MDT approach was applied. Suppressive antibiotics were the main treatment (N = 75, 44.4 %). First intention treatment of FRI involved surgery in only 94 (55.6 %) cases (Table 4). The most commonly used procedures included one-stage implant exchange (DAIEX, 11.2 %), two-stage DAIEX with a cement spacer (Masquelet technique, 10.7 %), debridement and initial fixation in previously non-stabilized fractures (DAIF, 9.5 %), and debridement with implant retention (DAIR, 9.5 %) (Fig. 2).
Bone void fillers included bone cement and grafts (10.7 %). No ceramic-based carriers were employed. Local antibiotics were delivered as powder without carrier (24.8 %), antibiotic-loaded cement (10.7 %), and antibiotic-impregnated bone graft (grafts harvested and mixed with antibiotics for 1 h before implantation in the same surgical procedure) (7.1 %). In implant exchange cases (N = 38), external fixation was the predominant stabilization method (N = 36, 94.7 %), including monoplanar (63.8 %) and biplanar (36.2 %). No circular frames were used. Soft tissue reconstruction used local or rotational flaps in 19 patients (11.3 %), with no free flaps (Table 4).
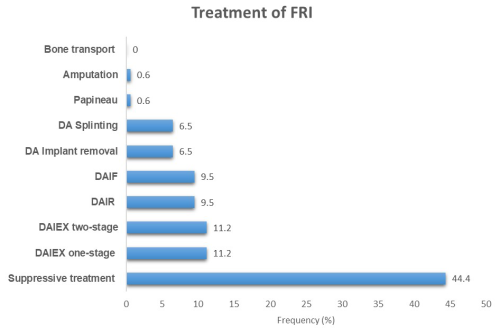
Figure 2Distribution of cases according to the treatment performed. DAIR: debridement, antibiotics, and implant retention; DAIEX: debridement, antibiotics, and implant exchange; DAIF: debridement, antibiotics, and initial fixation in previously non-stabilized fractures; DA: debridement, antibiotics.
Table 4Management of FRI.
DAIR: debridement, antibiotics, and implant retention; DAIEX: debridement, antibiotics, and implant exchange; DAIF: debridement, antibiotics, and initial fixation).
Prior to admission or surgery, 137 patients (81.1 %) were already on antibiotics. Mean antimicrobial duration was 11.1 ± 16.6 weeks. Excluding patients on suppressive therapy from this specific analysis, an appropriate antibiotic regimen was achieved in 13 patients (56.5 %) without an implant and in 20 patients (28.1 %) in whom an implant was retained or exchanged.
There was a significant association between the timing of FRI onset and treatment modality (surgical vs. non-surgical) (Fig. 3). Surgery was most frequent in delayed (73.9 %) and late FRI (76.5 %) than in early FRI (29.6 %). Notably, 60.3 % of patients who developed FRI without prior surgery underwent surgical treatment (p<0.0001).
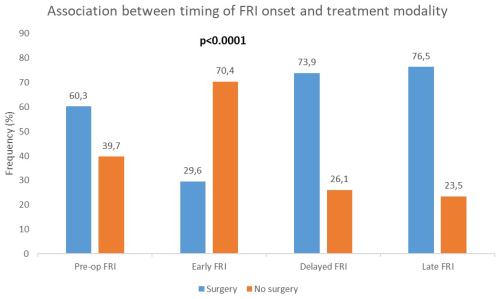
Figure 3Association between timing of onset of FRI and treatment modality. There was a statistically significant association between the timing of FRI onset and the treatment (surgical or non-surgical) (p<0.0001). Pre-op FRI: fracture-related infection diagnosed in the absence of previous surgical treatment.
3.5 Compliance with consensus guidelines
Treatment deviated from guidelines in 106 patients (62.7 %). Main reasons were inappropriate suppressive therapy (55.6 %), surgical indication not aligned with recommendations (29.2 %), or financial barriers preventing indicated treatment (15.2 %).
Since the publication of the international FRI consensus guidelines for the diagnosis and management of FRI, developed by experts from HICs, there has been limited evidence from LMICs addressing this issue. Consequently, the applicability and implementation of these recommendations in resource-limited settings remain uncertain. This study addresses this knowledge gap by providing updated, context-specific data on the diagnosis and management of FRIs in LMICs, thereby helping to answer a critical question: how applicable are the current international guidelines in these settings (Tissingh et al., 2022)?
4.1 Diagnostic criteria
FRI diagnosis relied on clinical confirmatory criteria in 97 % of cases, higher than in HIC studies at 47 % to 77 % (Vanvelk et al., 2023; Onsea et al., 2022; Buijs et al., 2022). Postoperative fever is often misattributed to malaria or urinary/respiratory infections, leading to antimalarial and empirical antibiotic use that can mask early-onset FRI (Fonkoué et al., 2024b). Other contributing factors may include patients' low awareness of early signs of FRI, systematic use of antibiotics via self-medication or empirical prescriptions, and delayed presentation. When clinical confirmatory signs do emerge, the initial response is frequently the prescription of antibiotics. Indeed, over 80 % of patients were already on antibiotics before admission, far higher than the 15.1 % reported by Onsea et al. (2022), highlighting the need for improved antibiotic stewardship (Onsea et al., 2022).
Microbiological confirmatory criteria were met in only 21.3 % of patients, and histological criteria were met in 1.2 % of patients (Metsemakers et al., 2018), although analysis was performed in 77.5 % of patients, with 80.2 % positivity. Recommended standards – 5 intraoperative deep tissue samples – were met in 0 % for quantity and 46.6 % for sampling method. With an average of 1.4 samples per patient (range: 1–3), we achieved a positivity rate of 80.2 %. In resource-limited settings where costs are borne by patients, 3 samples may be a practical compromise, yielding 85 % sensitivity versus 97 % for 5 samples (Dudareva et al., 2021), though some pathogens may be missed, particularly in polymicrobial infections (Corrigan et al., 2022). Histopathology may be justified only when clinical confirmation is lacking.
Efforts should focus on raising awareness and early suspicion of FRI. Local studies are needed to define algorithms based on suggestive clinical features and accessible investigations such as WBC, CRP, ESR, plain radiographs, and CT scans. Advanced imaging modalities, such as WBC scintigraphy and 18FDG-PET, which are not yet available, could help identify FRIs that may be missed due to the absence of confirmatory criteria; however, it should be noted that these are not mandatory in the FRI consensus definition. Both patients and healthcare providers must be educated to consider FRI in any delayed bone healing, especially after open fractures or prior surgery, regardless of timing.
4.2 Temporal classification of FRI
An important context-specific finding is that one-third of FRIs occurred in patients without prior surgery. This scenario is not addressed in the Willenegger and Roth classification (Willenegger and Roth, 1986) or current consensus guidelines (Metsemakers et al., 2018, 2020; Govaert et al., 2020; Marais et al., 2024a). The division of FRIs by time from injury is also controversial. McNally et al. (2022) found no correlation between time from injury and treatment outcome (McNally et al., 2022), and the new consensus FRI classification does not include time from injury (Alt et al., 2024). Likewise, classic strategies like DAIR and DAIEX are not literally applicable without implants; in such cases, we propose an adapted approach: DAIF (debridement, antibiotics, and initial fixation).
4.3 Management of FRI
A key observation was the absence of MDTs, despite most centers having necessary specialties except plastic surgery. Multidisciplinary care is well documented to improve outcomes (Rupp et al., 2023; Muller et al., 2022) without significant extra resources.
In two-thirds of cases, FRI treatment deviated from guidelines. A major deviation was the high rate (44.4 %) of suppressive antibiotics without surgery, likely reflecting limited surgical access, physician preference, limited education, and financial constraints. Among surgical treatments, DAIEX (23 %) was more common than DAIR (9.5 %), possibly due to delays in intervention. Surgical treatment was used in only 30 % of early infections, while 70 % of early infections were managed solely with antibiotics and dressings. This may be explained by the inherent challenge for surgeons in recognizing and acknowledging postoperative infectious complications and by the perception that an early return to the operating room represents a therapeutic failure. However, delayed DAIR can compromise implant salvage (Marais et al., 2024a; Baertl et al., 2024; Morgenstern et al., 2021; Vicenti et al., 2024) – a major concern in LMICs where implants are costly. Conversely, a South African study showed that in selected cases of FRI after intramedullary nailing, suppressive antibiotics alone achieved 95 % bone union and 98 % infection remission, despite DAIEX being indicated in these cases in HICs (Tsang et al., 2024). These findings suggest that in resource-limited settings, with stable constructs and adequate soft tissue coverage, not every FRI may require DAIR or DAIEX. Locally driven research is needed to clarify the role of non-surgical strategies in specific scenarios.
Achieving and maintaining adequate mechanical stability is crucial in managing fracture-related infection, as it promotes bone healing, reduces strain at the infection site, and enhances surgical debridement and systemic antibiotic efficacy (Foster et al., 2021). In this study, fixation relied mainly on external fixators, even during second-stage DAIEX procedures. Internal fixation was underutilized, likely due to concerns about reinfection and reoperation burden. This reluctance – aimed at minimizing risk – may compromise construct stability, which is a known factor in infection control. A paradigm shift is needed to encourage the safe use of internal fixation when indicated.
Long-acting local antibiotic carriers that allow single-stage treatment and safer internal fixation could be highly beneficial (Sliepen et al., 2022; McNally et al., 2022) but remain largely unavailable or unaffordable. Training in orthoplastic techniques is also critical for managing soft tissue defects and for improving FRI prevention – especially in open fractures – and treatment outcomes (Fonkoue et al., 2023a; Marais et al., 2024b; McNally et al., 2022).
4.4 Limitations
This study has limitations, including a relatively small sample, limited follow-up, and absence of outcome data. However, its primary aim was to evaluate the feasibility of applying consensus recommendations in a resource-limited setting rather than treatment outcomes. The prospective, multicenter design and the unique data provided enhance its relevance for informing future strategies and designing intervention trials.
This study found that the diagnosis of FRIs in low-resource settings is primarily based on clinical confirmatory criteria. As a result, the number of cases is likely underestimated. The recommendations regarding microbiological analysis – particularly the number and type of samples – appear difficult to implement in this context. Histopathological analysis, although accessible, is not yet routinely included in the diagnostic work-up for these patients. The FRI consensus definition is applicable in LMICs, particularly as it does not exclude patients without prior fracture fixation. Conventional surgical approaches (DAIR and DAIEX) are not applicable for one-third of patients who present with infections without having undergone prior surgery and thus without implant-related involvement. Therefore, DAIF may represent a relevant concept for LMIC-specific scenarios. Two-thirds of FRIs are managed outside international guidelines. This study provides key insights into the factors that could be targeted to improve the diagnosis and management of fracture-related infections in resource-limited settings. Further studies are warranted to identify outcome predictors in this specific context and to develop evidence-based, context-specific algorithms better suited to resource-constrained environments.
Raw data are available from the corresponding author upon reasonable request.
LF, ET, OC, and MM planned the study. CN, OKM, JSS, RD, and JB collected the data. LF and OKM evaluated guideline adherence. LF, CN, JSS, and RD processed the data and performed the statistical analyses. LF and ET wrote the paper. CN, OKM, JSS, RD, JB, OC, and MM reviewed the paper.
The contact author has declared that none of the authors has any competing interests.
This study was approved by the Institutional Review Board of the University of Yaoundé I, Cameroon (Ethical clearance No 0953/UY1/FMSB), and conducted following good clinical practice guidelines.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. The authors bear the ultimate responsibility for providing appropriate place names. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
During the preparation of this work, the author(s) used ChatGPT (OpenAI, San Francisco, CA) in order to assist with language refinement. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
This paper was edited by Willem-Jan Metsemakers and reviewed by two anonymous referees.
ABJIN (African Bone and Joint Infection Network): Management of fracture-related infection in low-resource settings, AO Alliance clinical guidelines, https://ao-alliance.org/wp-content/uploads/2025/02/clinical-guidelines_fracture-related-infections_2025.pdf (last access: 15 November 2025), 2025.
Alt, V., McNally, M., Wouthuyzen-Bakker, M., Metsemakers, W. J., Marais, L., Zalavras, C., and Morgenstern, M.: The FRI classification – A new classification of fracture-related infections, Injury, 55, 111831, https://doi.org/10.1016/j.injury.2024.111831, 2024.
Asim, R., Fuchs, C. J., and Summers, N. A.: Evaluating the duration of antimicrobial therapy for the treatment of orthopedic hardware infections, Microbiol Spectr, 12, e0126924, https://doi.org/10.1128/spectrum.01269-24, 2024.
Baertl, S., Rupp, M., and Alt, V.: The DAIR-procedure in fracture-related infection-When and how, Injury, 55 Suppl 6, 111977, https://doi.org/10.1016/j.injury.2024.111977, 2024.
Bezstarosti, H., Van Lieshout, E. M. M., Voskamp, L. W., Kortram, K., Obremskey, W., McNally, M. A., Metsemakers, W. J., and Verhofstad, M. H. J.: Insights into treatment and outcome of fracture-related infection: a systematic literature review, Arch Orthop Trauma Surg, 139, 61–72, https://doi.org/10.1007/s00402-018-3048-0, 2019.
Buijs, M. A. S., van den Kieboom, J., Sliepen, J., Wever, K. L. H., van Breugel, J. M., Hietbrink, F., IJpma, I. J., and Govaert, G. A. M.: Outcome and risk factors for recurrence of early onset fracture-related infections treated with debridement, antibiotics and implant retention: Results of a large retrospective multicentre cohort study, Injury, 53, 3930–3937, https://doi.org/10.1016/j.injury.2022.10.017, 2022.
Corrigan, R. A., Sliepen, J., Dudareva, M., IJpma, I. J., Govaert, G., Atkins, B. L., Rentenaar, R., Wouthuyzen-Bakker, M., and McNally, M.: Causative Pathogens Do Not Differ between Early, Delayed or Late Fracture-Related Infections, Antibiotics (Basel, Switzerland), 11, https://doi.org/10.3390/antibiotics11070943, 2022.
Depypere, M., Kuehl, R., Metsemakers, W. J., Senneville, E., McNally, M. A., Obremskey, W. T., Zimmerli, W., Atkins, B. L., and Trampuz, A.: Recommendations for Systemic Antimicrobial Therapy in Fracture-Related Infection: A Consensus From an International Expert Group, J Orthop Trauma, 34, 30–41, https://doi.org/10.1097/bot.0000000000001626, 2020a.
Depypere, M., Morgenstern, M., Kuehl, R., Senneville, E., Moriarty, T. F., Obremskey, W. T., Zimmerli, W., Trampuz, A., Lagrou, K., and Metsemakers, W. J.: Pathogenesis and management of fracture-related infection, Clin Microbiol Infect, 26, 572–578, https://doi.org/10.1016/j.cmi.2019.08.006, 2020b.
Dudareva, M., Barrett, L. K., Morgenstern, M., Atkins, B. L., Brent, A. J., and McNally, M. A.: Providing an Evidence Base for Tissue Sampling and Culture Interpretation in Suspected Fracture-Related Infection, J Bone Joint Surg Am, 103, 977–983, https://doi.org/10.2106/jbjs.20.00409, 2021.
Fonkoue, L., Muluem, O. K., Nana, T., Kong, D., Ngongang, O., Ngo Yamben, M., Tambekou, U., Tagakou, J., and Handy, D.: Outcome of a 2-stage management of open tibia fracture in a low-income country lacking plastic surgeons: A retrospective cohort study, Orthoplastic Surg 13, 25–30, 2023a.
Fonkoue, L., Tissingh, E. K., Muluem, O. K., Kong, D., Ngongang, O., Tambekou, U., Handy, D., Cornu, O., and McNally, M.: Predictive factors for fracture-related infection in open tibial fractures in a Sub-Saharan African setting, Injury, 54, 110816, https://doi.org/10.1016/j.injury.2023.05.047, 2023b.
Fonkoué, L., Muluem, K., Bessong, E., Ngongang, O., Mohamadou, G., Mebouinz, F., Ewolo, G., Ngo Yamben, M. A., Tiagadigui, G., Bahebeck, J., and Handy, D.: Incidence et facteurs prédictifs d'infection du site opératoire en chirurgie orthopédique et traumatologique propre à Yaoundé., Health Sci Dis, 25, 86–92, 2024a.
Foster, A. L., Moriarty, T. F., Trampuz, A., Jaiprakash, A., Burch, M. A., Crawford, R., Paterson, D. L., Metsemakers, W. J., Schuetz, M., and Richards, R. G.: Fracture-related infection: current methods for prevention and treatment, Expert Rev Anti Infect Ther, 18, 307–321, https://doi.org/10.1080/14787210.2020.1729740, 2020.
Foster, A. L., Moriarty, T. F., Zalavras, C., Morgenstern, M., Jaiprakash, A., Crawford, R., Burch, M. A., Boot, W., Tetsworth, K., Miclau, T., Ochsner, P., Schuetz, M. A., Richards, R. G., and Metsemakers, W. J.: The influence of biomechanical stability on bone healing and fracture-related infection: the legacy of Stephan Perren, Injury, 52, 43–52, https://doi.org/10.1016/j.injury.2020.06.044, 2021.
Govaert, G. A. M., Kuehl, R., Atkins, B. L., Trampuz, A., Morgenstern, M., Obremskey, W. T., Verhofstad, M. H. J., McNally, M. A., and Metsemakers, W. J.: Diagnosing Fracture-Related Infection: Current Concepts and Recommendations, J Orthop Trauma, 34, 8–17, https://doi.org/10.1097/bot.0000000000001614, 2020.
Kouassi, K. J. E., Manon, J., Fonkoue, L., Kodo, M., Detrembleur, C., and Cornu, O.: La prise en charge des fractures ouvertes de jambe dans une structure hospitalière en Côte d'Ivoire pose-t-elle problème et pourquoi?, Rev. Chir. Orthop Traumatol., 105, 654–658, 2019.
Fonkoué, L., Ewolo, G., Ngongang, O., Muluem, K., Tambekou, U., Mohamadou, G., and Bahebeck, J.: Post-Operative Fever in Adult Orthopaedics-Traumatology in Yaoundé: Incidence, Etiologies and Prognosis, Health Sci Dis, 25, 1–6, 2024b.
Marais, L. C., Zalavras, C. G., Moriarty, F. T., Kühl, R., Metsemakers, W. J., and Morgenstern, M.: The surgical management of fracture-related infection. Surgical strategy selection and the need for early surgical intervention, J Orthop, 50, 36–41, https://doi.org/10.1016/j.jor.2023.11.033, 2024a.
Marais, L. C., Hungerer, S., Eckardt, H., Zalavras, C., Obremskey, W. T., Ramsden, A., McNally, M. A., Morgenstern, M., and Metsemakers, W. J.: Key aspects of soft tissue management in fracture-related infection: recommendations from an international expert group, Arch Orthop Trauma Surg, 144, 259–268, https://doi.org/10.1007/s00402-023-05073-9, 2024b.
McNally, M., Corrigan, R., Sliepen, J., Dudareva, M., Rentenaar, R., F, I. J., Atkins, B. L., Wouthuyzen-Bakker, M., and Govaert, G.: What Factors Affect Outcome in the Treatment of Fracture-Related Infection?, Antibiotics (Basel, Switzerland), 11, https://doi.org/10.3390/antibiotics11070946, 2022.
Metsemakers, W. J., Moriarty, T. F., Morgenstern, M., Marais, L., Onsea, J., O'Toole, R. V., Depypere, M., Obremskey, W. T., Verhofstad, M. H. J., McNally, M., Morshed, S., Wouthuyzen-Bakker, M., and Zalavras, C.: The global burden of fracture-related infection: can we do better?, Lancet Infect Dis, 24(6), e386–e393, https://doi.org/10.1016/s1473-3099(23)00503-0, 2023.
Metsemakers, W. J., Morgenstern, M., Senneville, E., Borens, O., Govaert, G. A. M., Onsea, J., Depypere, M., Richards, R. G., Trampuz, A., Verhofstad, M. H. J., Kates, S. L., Raschke, M., McNally, M. A., and Obremskey, W. T.: General treatment principles for fracture-related infection: recommendations from an international expert group, Arch Orthop Trauma Surg, 140, 1013–1027, https://doi.org/10.1007/s00402-019-03287-4, 2020.
Metsemakers, W. J., Morgenstern, M., McNally, M. A., Moriarty, T. F., McFadyen, I., Scarborough, M., Athanasou, N. A., Ochsner, P. E., Kuehl, R., Raschke, M., Borens, O., Xie, Z., Velkes, S., Hungerer, S., Kates, S. L., Zalavras, C., Giannoudis, P. V., Richards, R. G., and Verhofstad, M. H. J.: Fracture-related infection: A consensus on definition from an international expert group, Injury, 49, 505–510, https://doi.org/10.1016/j.injury.2017.08.040, 2018.
Morgenstern, M., Kuehl, R., Zalavras, C. G., McNally, M., Zimmerli, W., Burch, M. A., Vandendriessche, T., Obremskey, W. T., Verhofstad, M. H. J., and Metsemakers, W. J.: The influence of duration of infection on outcome of debridement and implant retention in fracture-related infection, Bone Joint J, 103-b, 213–221, https://doi.org/10.1302/0301-620x.103b2.Bjj-2020-1010.R1, 2021.
Muller, Q., Gerber, F., Papadimitriou Olivgeris, M., Di Summa, P., Boillat Blanco, N., and Steinmetz, S.: [Multidisciplinary approach to fracture-related infection], Rev Med Suisse, 18, 2363–2370, https://doi.org/10.53738/revmed.2022.18.808.2363, 2022.
Obremskey, W. T., Metsemakers, W. J., Schlatterer, D. R., Tetsworth, K., Egol, K., Kates, S., and McNally, M.: ICM Orthopaedic Trauma Work Group* & ICM Orthopaedic Trauma Work Group*. Musculoskeletal infection in orthopaedic trauma: assessment of the 2018 International Consensus Meeting on Musculoskeletal Infection, J Bone Joint Surg Am, 102, https://doi.org/10.2106/JBJS.19.01070, 2020.
Onsea, J., Van Lieshout, E. M. M., Zalavras, C., Sliepen, J., Depypere, M., Noppe, N., Ferguson, J., Verhofstad, M. H. J., Govaert, G. A. M., FFA, I. J., McNally, M. A., and Metsemakers, W. J.: Validation of the diagnostic criteria of the consensus definition of fracture-related infection, Injury, 53, 1867–1879, https://doi.org/10.1016/j.injury.2022.03.024, 2022.
Prada, C., Bengoa, F., and Bhandari, M.: The management of fracture related infections: What practices can be supported by high-level evidence?, J Orthop Surg, 30, 10225536221119580, https://doi.org/10.1177/10225536221119580, 2022.
Rupp, M., Walter, N., Popp, D., Hitzenbichler, F., Heyd, R., Geis, S., Kandulski, M., Thurn, S., Betz, T., Brochhausen, C., and Alt, V.: Multidisciplinary Treatment of Fracture-Related Infection Has a Positive Impact on Clinical Outcome-A Retrospective Case Control Study at a Tertiary Referral Center, Antibiotics (Basel, Switzerland), 12, https://doi.org/10.3390/antibiotics12020230, 2023.
Saris, C. G., Bastianen, C. A., Swieten, E. C. A. M., and Wegdam, H. H.: Infection rate in closed fractures after internal fixations in a municipal hospital in Ghana, Trop Doct, 36, 233–235, https://doi.org/10.1258/004947506778604689, 2006.
Sliepen, J., Corrigan, R. A., Dudareva, M., Wouthuyzen-Bakker, M., Rentenaar, R. J., Atkins, B. L., Govaert, G. A. M., McNally, M. A., and IJpma, I. J.: Does the Use of Local Antibiotics Affect Clinical Outcome of Patients with Fracture-Related Infection?, Antibiotics (Basel, Switzerland), 11, https://doi.org/10.3390/antibiotics11101330, 2022.
Tissingh, E. K., Marais, L., Loro, A., Bose, D., Paner, N. T., Ferguson, J., Morgensten, M., and McNally, M.: Management of fracture-related infection in low resource settings: how applicable are the current consensus guidelines?, EFORT open Rev, 7, 422–432, https://doi.org/10.1530/eor-22-0031, 2022.
Tsang, S. J., van Rensburg, A. J., and Ferreira, N.: Is there a role for suppression of infection in managing fracture-related infection following intra-medullary nailing?, Injury, 55, 111602, https://doi.org/10.1016/j.injury.2024.111602, 2024.
Unsworth, A., Young, B., Ferguson, J., Scarborough, M., and McNally, M.: Local Antimicrobial Therapy with Combined Aminoglycoside and Vancomycin Compared to Aminoglycoside Monotherapy in the Surgical Management of Osteomyelitis and Fracture-Related Infection, Antibiotics (Basel, Switzerland), 13, https://doi.org/10.3390/antibiotics13080703, 2024.
Vanvelk, N., Van Lieshout, E. M. M., Onsea, J., Sliepen, J., Govaert, G., FFA, I. J., Depypere, M., Ferguson, J., McNally, M., Obremskey, W. T., Zalavras, C., Verhofstad, M. H. J., and Metsemakers, W. J.: Diagnosis of fracture-related infection in patients without clinical confirmatory criteria: an international retrospective cohort study, J Bone Joint Infect, 8, 133–142, https://doi.org/10.5194/jbji-8-133-2023, 2023.
Vicenti, G., Buono, C., Albano, F., Ladogana, T., Pesare, E., Colasuonno, G., Passarelli, A. C., and Solarino, G.: Early Management for Fracture-Related Infection: A Literature Review, Healthcare (Basel, Switzerland), 12, https://doi.org/10.3390/healthcare12131306, 2024.
Whiting, P. S., Galat, D. D., Zirkle, L. G., Shaw, M. K., and Galat, J. D.: Risk Factors for Infection After Intramedullary Nailing of Open Tibial Shaft Fractures in Low- and Middle-Income Countries, J Orthop Trauma,, 33, e234–e239, https://doi.org/10.1097/bot.0000000000001441, 2019.
Willenegger, H. and Roth, B.: [Treatment tactics and late results in early infection following osteosynthesis], Unfallchirurgie, 12, 241–246, https://doi.org/10.1007/bf02586085, 1986.