the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Multi-centre evaluation of Gram stain in the diagnosis of septic arthritis
Charlotte Smith
Robert J. Maloney
Deborah Wearmouth
Hemant Sharma
Kordo Saeed
Nusreen Ahmad-Saeed
Rachel Annett
Lucinda Barrett
Sara E. Boyd
Peter Davies
Harriet Hughes
Gwennan Jones
Laura Leach
Maureen Lynch
Deepa Nayar
Martin Marsh
Shanine Mitchell
Lynn Moffat
Luke S. P. Moore
Michael E. Murphy
Shaan Ashk O'Shea
Teresa Peach
Christina Petridou
Niamh Reidy
Ben Talbot
Catherine Aldridge
Gavin Barlow
Introduction: Gram stain of synovial fluid is a rapid test for the diagnosis of native joint septic arthritis. Single-centre studies have suggested Gram stain will miss a considerable proportion of patients who are subsequently synovial-fluid-culture-positive or polymerase chain reaction (PCR)-positive. The object of this study was to reassess Gram stain in a large, multi-centre cohort of patients from the United Kingdom (UK) and Ireland. Methods: The study was a retrospective analysis combining two large datasets. We defined septic arthritis microbiologically as at least one positive joint aspirate culture and/or PCR test. “Best case” and “worst case” definitions were applied depending on the likelihood organisms were true infecting pathogens. Results: Gram stain missed a high proportion of culture-/PCR-positive patients using both the best (74 % missed) and worst (81 % missed) case definitions. Using the best case definition, the sensitivity of Gram stain was 0.26, specificity 0.99, positive predictive value 0.84, negative predictive value 0.87, accuracy 0.87, and area under the receiver operator curve 0.62 (95 % CI 0.57 to 0.68, p<0.001). False positive Gram stains were infrequent (1 %). Age, joint involved, and other synovial fluid characteristics were less predictive of a positive culture/PCR than Gram stain. Conclusions: While a positive synovial fluid Gram stain should always be considered to indicate potential septic arthritis, a negative Gram stain, regardless of synovial fluid crystals or white cell count, should not be used to rule out septic arthritis. The value of Gram stain as an urgent out-of-hours test for septic arthritis is open to considerable debate.
- Article
(711 KB) - Full-text XML
- BibTeX
- EndNote
Septic arthritis of a native large joint commonly presents with non-specific features mimicking other acute joint conditions (Coakley et al., 2006). The incidence in the UK between 1998 and 2013 increased by 43 %, with an incidence of 7.8 per 100 000 by 2013 (Rutherford et al., 2016). It is crucial to have a timely, clear diagnosis as delayed management may result in sub-optimal treatment, joint damage, and even mortality (McBride et al., 2020). Long-term morbidity affects up to one-half of patients, with the need for joint replacement or other surgeries in some (Lauper et al., 2018; McBride et al., 2020).
The diagnosis of native septic arthritis is based on synthesizing the clinical presentation, radiological, and blood test findings alongside joint aspiration results. A microbiological diagnosis is achievable in approximately 70 % to 80 % of cases based on joint aspirate fluid or operative specimen culture (Cunningham et al., 2014; Holzmeister et al., 2021). Newer technologies, such as polymerase chain reaction (PCR), increase yield (Saeed et al., 2023). When one of these tests is positive for a recognized microbiological cause, it is diagnostic of septic arthritis. While most causative bacteria are Gram-positive, Gram-negatives occur in up to one of five cases, so an early microbiological diagnosis is vital to guide antibiotics (Arieli et al., 2021; McBride et al., 2020). In the UK and Ireland, the standard-of-care empiric treatment is intravenous (IV) flucloxacillin (or equivalent agent), which covers susceptible Gram-positive bacteria only (Coakley et al., 2006).
A synovial fluid Gram stain and culture are the “gold standard” microbiological tests (UK SMIs, 2023). The Gram stain is often performed rapidly and is generally considered to be an indicator of the likelihood of infection. Not all laboratories offer a 24 h service, meaning biomedical scientists may have to come in out of hours. Anecdotally, a negative Gram stain or the aspirate being positive for crystals is often considered an indication that infection is unlikely and can result in patient discharge. This can result in patient recall and is supported by previous studies that showed a high proportion of false negatives (e.g. 78 %) (Stirling et al., 2014). Given concerns about Gram stain in septic arthritis, the object of this study was to reassess its performance in a large multi-centre study in the UK and Ireland, where microbiological investigation is standardized by national standards (UK SMIs, 2023).
The study was a retrospective analysis involving eight large teaching hospitals in the UK and Ireland. Two datasets were combined, one from a multi-centre study of the BioFire PCR native joint infection panel (Saeed et al., 2023) and an unpublished dataset from a 1200-bed UK teaching hospital (no duplication in the two cohorts).
The national dataset was collected over 6 months at each site between March 2021 and March 2022. The single-centre dataset was collected during 26 June 2019 to 26 June 2021 (both continuous). All patients had a joint aspirate from a large native joint followed by microbiological assessment based on the UK's Standards for Microbiology Investigations (SMI) (UK SMIs, 2023). Prosthetic joints were excluded.
Data were audited and integrated into an Excel for Windows spreadsheet. Age and synovial fluid white cell count (WCC) were collected differently in the two studies such that when combined, patients were grouped into <18, 19–55, or >55 years for age and <10 and >10 white cells per high-powered field (HPF) for synovial fluid WCC. We did not collect patient data other than age and joint involved.
We defined septic arthritis microbiologically based on the presence of at least one positive direct or enrichment aspirate culture (when performed) and/or positive PCR test. Some organisms were considered potential contaminants rather than true pathogens, so we created two definitions: a “best case” definition based on organisms very likely to be true pathogens and a “worst case” definition based on best case organisms and those with a higher risk of being contaminants according to literature review and authors' experience (Table 1).
2.1 Statistical analyses
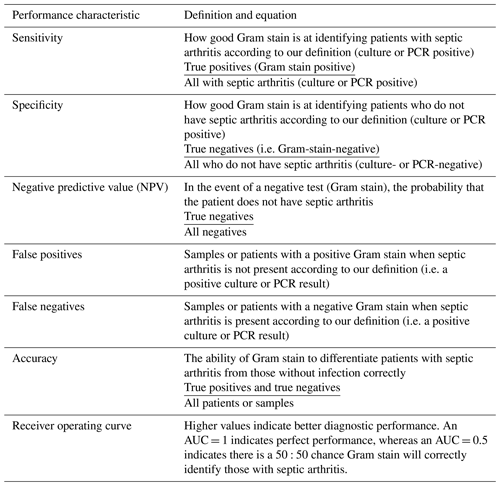
SPSS version 28 was used for statistical analyses. Descriptive statistics are given as medians or percentages with 95 % confidence intervals (CIs) where appropriate. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, the area under the receiver operator curve (AUROC), and associated 95 % CIs were calculated. The definitions of performance characteristics used are given in Table A1. Statistical associations between synovial fluid characteristics, the joint involved, and age were analysed using χ2 and multivariate binary logistic regression with a positive culture/PCR result (using both definitions in different models) as the dependent variable.
Only predictor variables with a p≤0.1 on univariate analyses were included in multivariate analyses. Logistic regression models were constructed with adjusted odds ratios, 95 % CIs, and p values from models considered the most clinically and statistically robust presented. P values < 0.05 were considered to indicate statistical significance. Analyses were performed by the total number of samples and by patient with results for both presented. When analysed by patient, only one sample was considered, either their index (first) sample if all others were negative by culture/PCR or the positive sample if the first sample was negative or the “most” positive sample if more than one sample was positive (e.g. if two organisms were identified in the first sample and only one in a second, the first sample was used).
Overall, our results, and conclusions thereof, were very similar regardless of whether analyses were by sample or patient or using the best case or worst case definitions of septic arthritis. Here we present the results by sample, and patient as appropriate, using the best case definition. To allow comparison, results by patient using the worst case definition are presented in the Appendix. The inclusion or exclusion of PCR results as part of our definitions did not change the results or conclusions thereof.
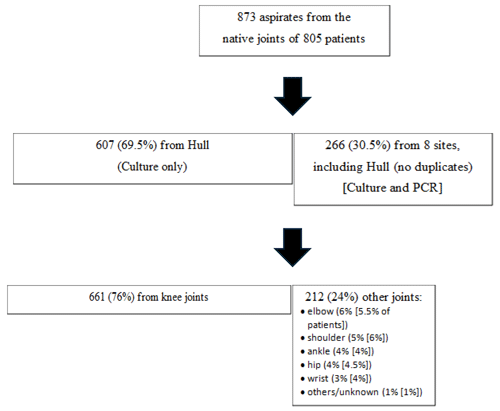
The number and characteristics of joint aspirates are in Fig. 1. The patient's exact age was only collected in the single centre study, with the mean age being 64 years (median = 67, range 2 to 99 years). Overall, 69.5 % (N=557/801) were >55 years old (65 % and 72 % in the two cohorts). Only 2.5 % of patients were <18 years old. A Gram stain was available for 98 % of samples (N=856), with 95 % being negative (N=812) and the same proportion negative in the two cohorts (95 %).
3.1 Synovial fluid culture and PCR, crystals, and white cell count
N=211 samples (24 %) had a positive direct or enrichment synovial fluid culture (also 24 % in those with a Gram result; 21 % at the largest site versus 31 % at other sites); 26 % of samples were positive by either culture and/or PCR. Of patients with at least one positive sample by culture/PCR, most were considered to have septic arthritis using the best case definition (N=136, 65 %); (64 %) in those with a Gram stain result.
Crystals were common: 25 % () of samples overall with 32 % () at the centre contributing most and 10 % (N=27/266) at other centres. One in nine patients (11 %) with positive crystals had septic arthritis by our best case definition. Of all samples, 3 % () had both positive crystals and a positive culture/PCR test by the best case definition.
Of all samples, 40 % () had a synovial fluid white cell count > 10 per HPF (54 % < 10, 6 % unknown/not done; 31 % and 46 % > 10 per HPF in the two datasets). Of patients with a WCC > 10 per HPF (N=308), 27 % were deemed to reflect septic arthritis by the best case definition versus 10 % in those with a WCC < 10 (N=446).
3.2 Relationship between Gram stain and culture/PCR
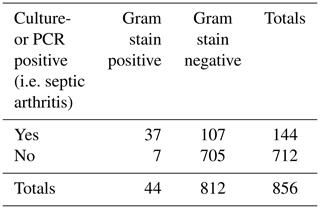
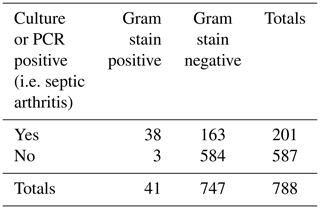
Of samples with a positive Gram stain (N=44), culture or PCR was positive in 84 % (N=37) using the best case definition. Of samples with a negative Gram stain (N=812), culture/PCR was positive in 107 (13 %) using the best case definition.
Of samples with a positive culture/PCR (N=144) using the best case definition, the Gram stain was positive in 26 % (N=37). Of samples with a negative culture/PCR (N=712), the Gram stain was positive in 1 % (N=7) (false positives) using the best case definition.
Of patients who had a Gram-positive organism cultured or by PCR (best case definition), 24.5 % had a positive Gram stain test versus 10.5 % of patients with Gram-negative organisms and 12.5 % in those with both a Gram-positive and a Gram-negative organism (χ2=2.35, P>0.3).
3.3 Performance characteristics
Using the best case definition by sample, the sensitivity of Gram stain was 0.26, specificity 0.99, positive predictive value (PPV) 0.84, negative predictive value (NPV) 0.87, and accuracy 0.87; see Table 2. By patient, the same performance characteristics were 0.27, 0.99, 0.85, 0.87, and 0.87, respectively. The AUROC was 0.62 (95 % CI 0.57 to 0.68, p<0.001) using the best case definition.
3.4 Univariate and multivariate analyses of Gram stain by patient
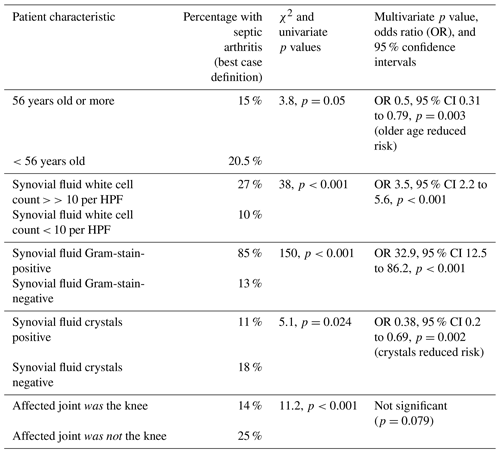
A summary of analyses is in Table 3. In multivariate analyses (binary logistic regression) with septic arthritis, using the best case definition, as the dependent variable and variables identified in univariate analyses as potential predictors, older age and crystals but not the joint being the knee, were found to be associated with a reduced likelihood of septic arthritis. In contrast, a synovial fluid WCC ≥ 10 per HPF and Gram stain positivity were associated with an increased risk of septic arthritis: Hosmer–Lemeshow test χ2=6.4, p=0.49. There was a statistically significant association (χ2=6.6, p=0.01) between older age and whether the joint aspirate was from the knee. Removing one or other or both from the model improved the model's statistical performance (Hosmer–Lemeshow test) while strengthening the lack of association between the joint involved and septic arthritis when this variable was retained. This did not change our overall results or conclusions.
3.5 Microbiology
The most identified bacteria are in Table 4. Approximately 1 in 15 patients (6.5 %) with a positive culture/PCR had >1 organism identified. The proportion of patients with a positive Gram stain was much higher in patients with septic arthritis according to the best case definition versus patients with organisms deemed less likely to represent true septic arthritis (27 % (best case) versus 4 % (worse case) versus 0.5 % (culture-negative), χ2=152, P<0.001). A similar association was identified for high synovial fluid WCC (≥10 per HPF) (66 % (best case) versus 28 % (worse case) versus 37 % (culture-negative), χ2=40, P<0.001).
Table 4Bacteria identified by culture or PCR (i.e. all organisms identified regardless of whether best or worst case organisms).
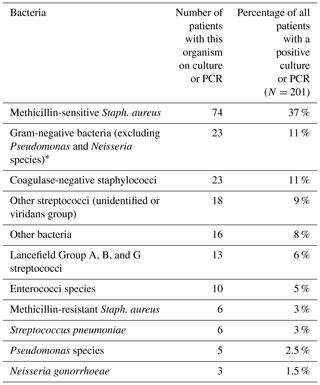
* Gram-negative bacteria: Citrobacter species (0.5 %) Escherichia coli (3.5 %), Enterobacter species (0.5 %), Haemophilus parainfluenzae (1.5 %), Kingella kingae (0.5 %), Klebsiella pneumoniae (1.5 %), Moraxella species (0.5 %), Proteus species (2 %), Providencia species (0.5 %), and Serratia species (0.5 %).
Our key finding is that a negative synovial fluid Gram stain, regardless of other sample characteristics or patient age or joint involved, or the nature of the microbiological definition, cannot rule out septic arthritis. Indeed, Gram stain will miss most (74 %) subsequently culture-/PCR-positive synovial fluid specimens. In contrast, the vast majority of patients with a positive Gram stain will have a positive culture/PCR; false positives are rare. The accuracy of Gram stain and culture/PCR can depend on factors such as operator experience or error and patient exposure to antibiotics, although such factors reflect the realities of real life. While a positive Gram stain is undoubtedly useful, in our experience it tends to be negative Gram stains, particularly when crystals are present, that are more likely to lead to poor clinical decisions and under-intervention.
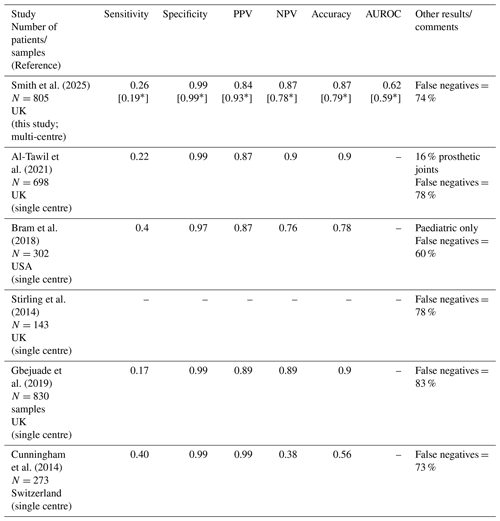
A summary of the performance characteristics of Gram stain for the diagnosis of native joint septic arthritis based on large (N≥100 patients) published studies, identified during a PubMed search using the keywords septic, infective, infectious, arthritis, arthropathy, synovial, and Gram stain, is in Table 5 (studies that mixed native and prosthetic joint data when they could not be extracted separately were excluded). We identified five studies in addition to this study (3051 patients). Results were similar across studies with sensitivity ranging from 0.17 to 0.4, specificity 0.97 to 0.99, PPV 0.84 to 0.99, NPV 0.38 to 0.9, and accuracy 0.56 to 0.9. False negatives were high in all studies (60 % to 83 %; 73 % to 83 % when the one paediatric study is excluded). Given the large number of patients across studies and consistency of findings, we have a high level of confidence in our conclusions. All studies were performed in high-income settings.
In keeping with others (Prior-Español et al., 2019), our results show synovial fluid with both crystals and positive culture/PCR is relatively common. Crystals should not rule out septic arthritis, even though this was predictive in multivariate analyses. Likewise, synovial fluid WCC at a cut-off of ≥10 per HPF was much less predictive of septic arthritis than Gram stain. In their systematic review, Walinga et al. (2021) found a synovial fluid WCC with a cut-off of 50 000 mm−3 was the most applied in studies with a sensitivity from 53 % to 100 % and specificity 66 % to 97 %. The proportion of synovial polymorphonuclear cells with cut-offs ranging from 75 % to 95 % showed a sensitivity from 42 % to 100 % and specificity 54 % to 94 %. Holzmeister et al. (2021) developed a calculator using 281 patients to help in the diagnosis of knee septic arthritis. Synovial WCC ≥ 30 000 µL had the highest odds ratio (91) compared to other statistically significant predictors: Gram stain positivity (21.5), duration of pain > 2 d (7), prior septic arthritis (5), knee effusion (5), and synovial fluid crystals (0.1) (Holzmeister et al., 2021). As far as we know, this calculator has not been validated in other large cohorts or joints.
We found older age significantly less associated with septic arthritis. This is likely to be due to a higher proportion of older adults presenting with non-infective causes of an acute joint. The joint involved (knee versus others) was not useful in predicting subsequent culture/PCR result. Staphylococcus aureus was the most common organism identified in patients with a positive culture/PCR, with more than three-quarters of culture-/PCR-positive patients having Gram-positive bacteria. It is worth noting that 15 % of patients with a positive culture/PCR (all organisms; 23 % in those with a best case organism) had Gram-negative bacteria, in keeping with the published literature (McBride et al., 2020) and highlighting the importance of identifying patient risk factors for septic arthritis caused by Gram-negative bacteria to guide empiric antibiotic therapy. Approximately double the proportion of patients had a positive Gram stain when only Gram-positive bacteria were identified, although this was not statistically significant.
Walinga et al. (2021) also found serum erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin, and synovial fluid Gram stain to be useful tests for septic arthritis, although all had variable performance, and the quality and the size of studies were generally low; this study is larger than the largest study in that review. Gram stain (N=5 studies) had a sensitivity and specificity ranging from 27 % to 81 % and 99 % to 100 %, respectively. Dey et al. (2023) performed a systematic review of the diagnosis of the acute joint but were unable to meta-analyse studies that had assessed Gram stain. Of other synovial fluid markers, leucocyte esterase had the highest pooled sensitivity (0.94 (95 % 0.70 to 0.99); specificity 0.74 (0.67 to 0.81); AUROC 0.78), while lactate (≥10 mmol L−1) had the highest pooled specificity (0.99 (0.96 to 1.0); sensitivity 0.36 (0.22 to 0.53); AUROC 0.85), and both tumour necrosis factor-α (36 pg mL−1) and procalcitonin (0.5 µg L−1) had the highest pooled AUROCs (0.93; TNFα sensitivity 0.86 (0.49 to 0.97), specificity 0.88 (0.54 to 0.98) and procalcitonin sensitivity 0.67 (0.26 to 0.92), specificity 0.93 (0.84 to 0.97)). Emerging technologies that could be used instead of Gram stain in the rapid diagnosis of septic arthritis need further evaluation.
Limitations
As with all observational studies, there is a risk of bias and confounding. Although the two amalgamated cohorts were similar, some characteristics were different, which likely reflects factors such as study design, local clinical practices, and differences in patient populations. Although we used pragmatic microbiological definitions, most published studies to date have done similarly, and there is no accepted “gold standard” definition of septic arthritis. It is also important to acknowledge that culture-/PCR-negative septic arthritis is well recognized.
Likewise, we used best and worst case definitions to reflect that some results may be due to specimen contamination. We acknowledge that by classifying all coagulase-negative staphylococci (CoNegS) in the worst case definition, organisms such as Staphylococcus lugdunensis that are more likely to be pathogens may have been misclassified. Not all laboratories in this study identified CoNegS to species level, but of those that did only Staphylococcus capitis (1 % of all organisms identified), Staphylococcus epidermidis (0.5 %), Staphylococcus hominis (0.5 %), and Staphylococcus warneri (0.5 %) were identified. Some may argue that all positive synovial fluid culture or PCR results are clinically significant. Without the context of detailed clinical data, we cannot confirm or refute this, but our approach is supported by a much higher proportion of worst case organisms growing on enrichment only (82 %), which is more likely to be associated with contamination, versus best case organisms (24 %). Despite these limitations, this is the largest such multi-centre study to date, performed within health systems with national standards for microbiological investigations and that is consistent with, supports, and adds to previous research.
In this large multi-centre study, Gram stain missed most patients with a synovial fluid aspirate subsequently positive by culture or PCR. In keeping with previous work, a negative Gram stain, regardless of other synovial fluid characteristics, the joint involved, or patient age, does not rule out septic arthritis.
A1 Additional results presented by patient and using the worst case definition as appropriate
A Gram stain was available for 98 % of patients (), with 93 % being negative ().
A2 Synovial fluid culture and PCR, crystals, and white cell count
In total, 24 % of patients had a positive direct or enrichment synovial fluid culture. Of patients with at least one positive sample by culture or PCR, most (64 %) were considered to have septic arthritis using the worst case definition () in those with a Gram stain result.
Crystals were identified in 24 % () of patients, with 31.5 % at the centre contributing most and 10 % at other centres. One in six patients (17 %) with positive crystals had septic arthritis by our worst case definition; 4.5 % () of samples had both positive crystals and a positive culture/PCR test by the worst case definition, which is 4 % of patients ().
Of patients with a WCC > 10 per HPF (N=308), 33 % were deemed to reflect septic arthritis by the worst case definition versus 21 % in those with a WCC < 10 (N=446).
A3 Relationship between Gram stain and culture/PCR
Of patients with a positive Gram stain (N=41), culture or PCR was positive in 85 % (N=35) using the best case definition and 93 % of samples and patients using the worst case definition. Of patients with a negative Gram stain, culture/PCR was positive in 94 (13 %) using the best case definition (22 % () using the worst case definition).
Of samples with a positive culture/PCR (N=218) using the worst case definition, Gram stain was positive in 19 % (N=41); (27 %) and (19 %) of patients using the best case and worst case definitions, respectively. Of samples with a negative culture/PCR (N=712), Gram stain was positive in 0.5 % () (false positives) using the worst case definition and (1 %) and (0.5 %) of patients using the best case and worst case definitions, respectively.
Of patients who had a Gram-positive organism cultured or identified by PCR (worst case organisms), 31 % had a positive Gram stain test versus 12.5 % of patients with Gram-negative organisms and 12.5 % in those with both a Gram-positive and a Gram-negative organism (χ2=3.3, P>0.2).
A4 Performance characteristics
Using the worst case definition, performance characteristics were similar whether analysed by sample (or patient): sensitivity 0.19 (0.19), specificity 0.99 (0.99), PPV 0.93 (0.93), NPV 0.78 (0.78), and accuracy 0.79 (0.79). The AUROC was 0.59 (0.54 to 0.64) by patient using the worst case definition.
A5 Univariate and multivariate analyses of Gram stain
For multivariate analyses using the worst case definition, results were similar compared to those using the best case definition, although age was not significantly associated with subsequent septic arthritis by univariate analysis. Hospital site was significantly associated with septic arthritis in both univariate and multivariate analyses (Hull 20 %, other sites 38 % septic arthritis, p<0.001 univariate and multivariate), as was septic arthritis being more likely to be a non-knee joint (p=0.046). A multivariate model without hospital site improved performance of the model and again found no statistical association between the joint involved and septic arthritis. Again, these analyses did not change our overall results or conclusions.
The data are currently being used for ongoing projects – however, requests to access the data are welcomed and can be done by sending an e-mail to gavin.barlow@york.ac.uk.
GB, CS, RM, and DW developed the research question, designed the study, performed the statistical analyses, and interpreted the results. GB and CS prepared the manuscript with editorial contributions from all co-authors. All co-authors otherwise contributed to laboratory analyses and/or data collection at their respective centres and have agreed to publication.
Kordo Saeed has received research grants and speaker fees from Pfizer, Thermo Fisher, and Menarini. Luke S. P. Moore has consulted for and received speaker fees from bioMerieux, Pfizer, Eumedica, Shionogi, Pulmocide, Umovis Labs, DNA Electronics, Kent Pharma, Sumitovant, Qiagen, and Gilead and received research grants from the UK National Institute for Health Research (NIHR), LifeArc, North West London Pathology, Shionogi, CW+ charity, and the Healthcare Infection Society. Sara E. Boyd held research funding through an MRC RCUK/UKRI Innovation Fellowship (MR/R016895/1) and the North West MRC Scheme in Clinical Pharmacology (MR/N025989/1). She received research support from Roche Pharma and has consulted for and received speaker fees from Sumitovant and Shionogi. Catherine Aldridge received speaker fees from bioMerieux. Gavin Barlow has received consultancy and/or writing fees in the last 3 years from UCB Pharma, Slough, UK (antimicrobials); bioMerieux, UK (septic arthritis diagnostics); Pfizer, UK (pneumococcal vaccine); and AdvanzPharma, UK (cellulitis). Deepa Nayar has received speaker fees from Tillotts and Biocomposites and unconditional education grants to attend meetings from Shionogi, Tillotts, and Biocomposites. Martin Marsh has received research funding or support and/or consultation fees from Pfizer, Shionogi, Tillotts, Biomerieux, and Microplate Dx. Hemant Sharma has received research grants from NIHR, Smith & Nephew, Orthofix, and B. Braun. He has also done paid consultancy for Orthofix, Smith & Nephew, and Biocomposites alongside being the past president and treasurer of the British Limb Reconstruction Society and ex chair of the Limb Reconstruction Committee (SICOT). Harriet Hughes has had reimbursement for educational meetings from Zimmer Biomet.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
All sites obtained approval via their research ethics and/or service evaluation or quality improvement governance boards as appropriate to the site. No patient-identifying data were collected with data stored in accordance with the UK's Data Protection Act 2018 and the General Data Protection Regulation (GDPR).
This paper was edited by Parham Sendi and reviewed by three anonymous referees.
Al-Tawil, K., Quiney, F., Pirkis, L., Birkett, N., and Rooney, A.: Gram stain microscopy in septic arthritis, Acta Orthop. Belg., 87, 553–556, 2021.
Arieli, M. M., Fowler, M. L., Lieber, S. B., Shmerling, R. H., and Paz, Z.: The profile of the causative organisms which lead to septic arthritis of native joints over the last two decades in a single tertiary medical center in the east coast of the United States, Int. J. Clin. Pract., 75, e15003, https://doi.org/10.1111/ijcp.15003, 2021.
Bram, J. T., Baldwin, K. D., and Blumberg, T. J.: Gram Stain is Not Clinically Relevant in Treatment of Pediatric Septic Arthritis, J. Pediatr. Orthop., 38, e536–e540, https://doi.org/10.1097/BPO.0000000000001226, 2018.
Coakley, G., Mathews, C., Field, M., Jones, A., Kingsley, G., Walker, D., Phillips, M., Bradish, C., McLachlan, A., Mohammed, R., and Weston, V.: British Society for Rheumatology Standards, Guidelines and Audit Working Group, BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults, Rheumatology, 8, 1039–1041, https://doi.org/10.1093/rheumatology/kel163a, 2006.
Cunningham, G., Seghrouchni, K., Ruffieux, E., Vaudaux, P., Gayet-Ageron, A., Cherkaoui, A., Godinho, E., Lew, D., Hoffmeyer, P., and Uçkay, I.: Gram and acridine orange staining for diagnosis of septic arthritis in different patient populations, Int. Orthop., 38, 1283–1290, https://doi.org/10.1007/s00264-014-2284-3, 2014.
Dey, M., Al-Attar, M., Peruffo, L., Coope, A., Zhao, S. S., Duffield, S., and Goodson, N.: Assessment and diagnosis of the acute hot joint: a systematic review and meta-analysis, Rheumatology, 62, 1740–1756, https://doi.org/10.1093/rheumatology/keac606, 2023.
Gbejuade, H., Elsakka, M., and Cutler, L.: How well does synovial fluid gram staining correlate with cultures in native joint infections?, Orthop. Rev., 11, 8156, https://doi.org/10.4081/or.2019.8156, 2019.
Holzmeister, A., Frazzetta, J., Yuan, F. F. N., Cherones, A., Summers, H., Cohen, J., and Lack, W. D.: Evaluation for septic arthritis of the native adult knee is aided by multivariable assessment, Am. J. Emerg. Med., 46, 614–618, https://doi.org/10.1016/j.ajem.2020.11.048, 2021.
Lauper, N., Davat, M., Gjika, E., Müller, C., Belaieff, W., Pittet, D., Lipsky, B. A., Hannouche, D., and Uçkay, I.: Native septic arthritis is not an immediate surgical emergency, J. Infect., 77, 47–53, https://doi.org/10.1016/j.jinf.2018.02.015, 2018.
McBride, S., Mowbray, J., Caughey, W., Wong, E., Luey, C., Siddiqui, A., Alexander, Z., Playle, V., Askelund, T., Hopkins, C., Quek, N., Ross, K., Orec, R., Mistry, D., Coomarasamy, C., and Holland, D.: Epidemiology, Management, and Outcomes of Large and Small Native Joint Septic Arthritis in Adults, Clin. Infect. Dis., 70, 271–279, https://doi.org/10.1093/cid/ciz265, 2020.
Prior-Español, Á., García-Mira, Y., Mínguez, S., Martínez-Morillo, M., Gifre, L., and Mateo, L.: Coexistence of septic and crystal-induced arthritis: A diagnostic challenge. A report of 25 cases, Reumatol. Clin., 15, e81–e85, https://doi.org/10.1016/j.reuma.2017.12.015, 2019.
Rutherford, A. I., Subesinghe, S., Bharucha, T., Ibrahim, F., Kleymann, A., and Galloway, J. B.: A population study of the reported incidence of native joint septic arthritis in the United Kingdom between 1998 and 2013, Rheumatology, 55, 2176–2180, https://doi.org/10.1093/rheumatology/kew323, 2016.
Saeed, K., Ahmad-Saeed, N., Annett, R., Barlow, G., Barrett, L., Boyd, S. E., Boran, N., Davies, P., Hughes, H., Jones, G., Leach, L., Lynch, M., Nayar, D., Maloney, R. J., Marsh, M., Milburn, O., Mitchell, S., Moffat, L., Moore, L. S. P., Murphy, M. E., O'Shea, S. A., O'Sullivan, F., Peach, T., Petridou, C., Reidy, N., Selvaratnam, M., Talbot, B., Taylor, V., Wearmouth, D., and Aldridge, C.: A multicentre evaluation and expert recommendations of use of the newly developed BioFire Joint Infection polymerase chain reaction panel, Eur. J. Clin. Microbiol. Infect. Dis., 42, 169–176, https://doi.org/10.1007/s10096-022-04538-w, 2023.
Stirling, P., Faroug, R., Amanat, S., Ahmed, A., Armstrong, M., Sharma, P., and Qamruddin, A.: False-negative rate of gram-stain microscopy for diagnosis of septic arthritis: suggestions for improvement, Int. J. Microbiol., 2014, 830857, https://doi.org/10.1155/2014/830857, 2014.
UK SMIs – UK Standards for Microbiology Investigations: Standard for microbiology investigations (UK SMI), https://www.gov.uk/government/collections/standards-for-microbiology-investigations-smi (last access: 16 October 2023), 2023.
Walinga, A. B., Stornebrink, T., Langerhuizen, D. W. G., Struijs, P. A. A., Kerkhoffs, G. M. M. J., and Janssen, S. J.: What are the best diagnostic tests for diagnosing bacterial arthritis of a native joint?: a systematic review of 27 studies, Bone Joint J., 103-B, 1745–1753, https://doi.org/10.1302/0301-620X.103B12.BJJ-2021-0114.R1, 2021.