the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Diagnostic accuracy of alpha-defensin ELISA and lateral flow assays for periprosthetic joint infection: a systematic review and meta-analysis
Benjamin R. Paul
Alex Soriano
Andy Miller
Thorsten M. Seyler
Background: Alpha-defensin (AD) is a synovial biomarker that can be used in the diagnosis of periprosthetic joint infection (PJI). Two testing modalities are available: the laboratory-based enzyme-linked immunosorbent assay (ELISA) and the point-of-care (POC) lateral flow (LF) assay. Although both assays have been incorporated into modern PJI diagnostic algorithms, their comparative diagnostic accuracy remains incompletely defined. Methods: A systematic review and meta-analysis were conducted following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Embase, and Cochrane databases were searched from 1 January 2000 to 1 February 2024. Studies using contemporary PJI definitions were included. Eligible studies evaluated the diagnostic performance (sensitivity and specificity) of AD-ELISA or AD-LF in patients undergoing evaluation for suspected PJI. Pooled sensitivity and specificity were calculated, and subgroup analyses compared AD assays to traditional synovial markers such as leukocyte count (LC) and polymorphonuclear percentage (PMN %). Results: A total of 51 studies met inclusion criteria. Reported sensitivity and specificity varied widely across studies, with median values of 0.86 and 0.97 for AD-ELISA and 0.84 and 0.97 for AD-LF. Pooled estimates, derived from studies reporting confidence intervals, demonstrated a sensitivity and specificity of 87.8 % (95 % CI, 81.2 %–94.3 %) and 97.9 % (95 % CI, 96.5 %–99.2 %) for AD-ELISA and of 81.8 % (95 % CI, 76.0 %–87.5 %) and 97.0 % (95 % CI, 95.9 %–98.2 %) for AD-LF, respectively. Compared with traditional synovial leukocyte count and PMN %, both assays demonstrated comparable or superior specificity, particularly for AD-ELISA. Risk of bias was generally low across included studies. Conclusion: Both AD assays demonstrate high specificity in diagnosing PJI, but AD-ELISA offers superior sensitivity compared to AD-LF and traditional synovial markers. Given variability in the underlying diagnostic criteria for PJI, these results should be interpreted within the context of differing reference standards. These findings support the continued use of AD-ELISA as a valid diagnostic modality.
- Article
(4972 KB) - Full-text XML
- BibTeX
- EndNote
Periprosthetic joint infection (PJI) is a rare but severe complication following total joint arthroplasty (TJA) and is defined by one of several diagnostic criteria commonly used by different organizations, including definitions by the Musculoskeletal Infection Society (MSIS) 2013 (Parvizi and Gehrke, 2014), the International Consensus Meeting (ICM) 2018 (Parvizi et al., 2018), the European Bone and Joint Infection Society (EBJIS) 2021 (McNally et al., 2021), and the Infectious Disease Society of America (IDSA) 2013 (Osmon et al., 2013). These diagnostic criteria are generally a combination of clinical signs, laboratory markers, microbial cultures, histopathologic findings, and intraoperative assessments, with each set of guidelines applying specific thresholds and scoring systems to establish a diagnosis.
Despite advances in diagnostic tools, accurately identifying PJI remains a challenge. No single test offers perfect sensitivity or specificity or has been universally accepted as the diagnostic gold standard. Traditional infection markers, including serum C-reactive protein (CRP), synovial white blood cell count, and intraoperative cultures, are restrained by variable performance, false negatives, and delayed turnaround time (Kheir et al., 2018; Quinlan and Jennings, 2023). Additionally, classic clinical features associated with infection, including fever and leukocytosis, are frequently absent in chronic or low-grade PJI (Slullitel et al., 2018). The diagnostic challenge of diagnosing PJI is further compounded by the significant clinical overlap between clinical presentations of PJI and aseptic failure. Both conditions may present with significant joint pain, swelling, stiffness, and radiographic evidence of implant loosening, making it difficult to differentiate between infectious versus non-infectious etiologies (Patel et al., 2016). This overlap can delay diagnosis and treatment, which ultimately increases the risk for adverse patient outcomes.
In response to these diagnostic challenges, there has been growing interest in the identification of novel biomarkers to improve diagnostic accuracy of PJI detection. Among these, alpha-defensin (AD) has emerged as a promising tool in the diagnostic workup of PJI. AD is an antimicrobial peptide that is released by neutrophils into the synovial fluid upon encountering bacterial pathogens (Lehrer and Ganz, 1992). Unlike conventional markers that may be elevated in the setting of sterile inflammation or mechanical irritation, AD is believed to be more specific to true infection (Deirmengian et al., 2005; Hubert et al., 2024; Lehrer and Ganz, 1992). Two commercially available test formats exist: the laboratory-based alpha-defensin enzyme-linked immunosorbent assay (AD-ELISA) and the point-of-care (POC) alpha-defensin lateral flow (AD-LF) test. Both have demonstrated favorable sensitivity and specificity in prior studies. However, an updated synthesis of the current evidence is necessary to more clearly define and compare the diagnostic performance of these two assays in TJA.
The purpose of this systematic review and meta-analysis was to synthesize the available high-quality evidence on the diagnostic accuracy of both AD-ELISA and AD-LF in the diagnosis of PJI. This study also aimed to explore heterogeneity across the included studies and assess how diagnostic performance varies by test type, study design, and patient population characteristics. Additionally, stratified analyses have compared AD assays with traditional synovial biomarkers such as leukocyte count and polymorphonuclear leukocyte percentage (PMN %), limiting inclusion to studies that excluded these markers from their diagnostic reference standard to reduce incorporation bias.
2.1 Search strategy
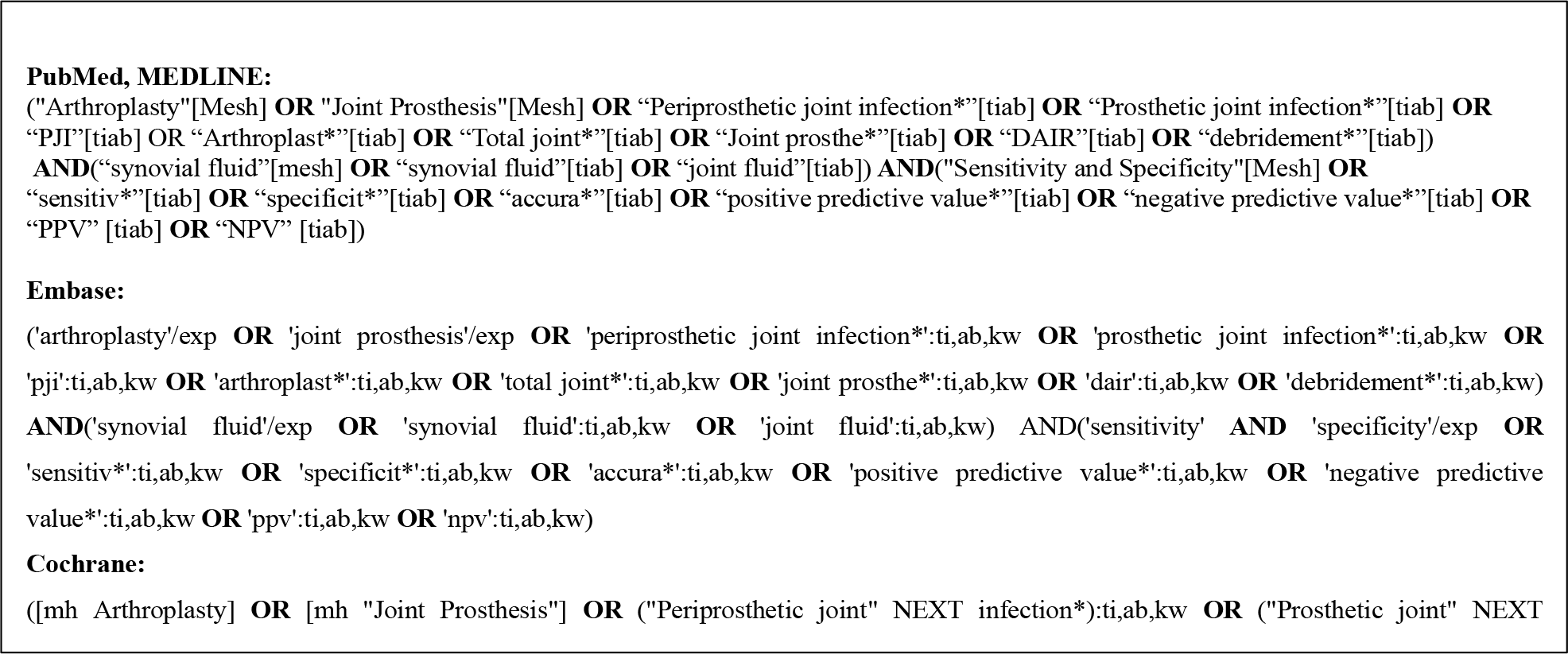
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) methodology was employed (Page et al., 2021). Studies that were published from 1 January 2000 to 1 February 2024 were queried using the following medical databases: PubMed, Embase, and Cochrane. The search strategy employed a combination of the following keywords: “Arthroplasty OR Joint Prosthesis OR Periprosthetic joint infection OR Prosthetic joint infection OR PJI OR Arthroplast OR Total joint OR Joint Prosthe or DAIR or debridement AND synovial fluid OR joint fluid AND Sensitivity and Specificity OR sensitiv OR specificit OR accura OR positive predictive value OR negative predictive value OR PPV OR NPV”. See Appendix A for search criteria specific to each medical database used.
2.2 Study assessment and eligibility criteria
The studies included in the meta-analysis were independently selected by two authors. Initial screening involved titles and abstracts, followed by full-text review for studies that met the inclusion criteria or where eligibility was unclear. Any discrepancies regarding study eligibility were resolved by a third author. Included studies met the following criteria: (a) evaluation of AD for diagnosing PJI using either quantitative ELISA or qualitative lateral flow in synovial fluid, (b) clear reporting of sensitivity and specificity, (c) PJI diagnosis based on a recognized reference standard (MSIS 2013, ICM 2018, EBJIS 2021, or IDSA 2013), and (d) publication in English.
2.3 Data extraction and quality assessment
The number of PJIs, number of controls, sensitivity, specificity, and confidence intervals were extracted from each study. Quality assessment of each study was conducted using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool (Whiting et al., 2011). The tool evaluates four domains of potential bias: (a) patient selection, (b) index test, (c) reference standard, and (d) flow and timing. Each domain was independently rated as having a low, unclear, or high risk of bias. Discrepancies were resolved through discussion with a third author.
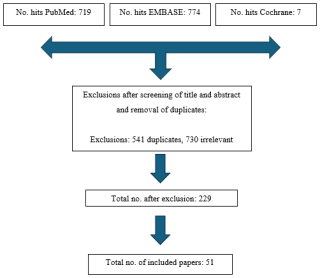
A flow diagram of the study selection is demonstrated in Fig. 1. The initial search yielded 719 records from PubMed, 774 from Embase, and 7 from Cochrane, for a total of 1500 citations from 1 January 2000 to 1 February 2024. After removal of 541 duplicate entries and exclusion of 730 articles based on the abstract and title not meeting the study design criteria, 229 studies remained for full-text review. These exclusions were most commonly due to the article being a review or meta-analysis rather than primary data, commentaries, non-English articles, or studies that did not apply an accepted definition of PJI. After full-text review, 51 studies specific to alpha-defensin met the eligibility criteria and were included in the final analysis.
2.4 Statistical analysis
Statistical analysis was performed using Microsoft Excel (Microsoft Corporation, Redmond, Washington) and R software (version 4.4.3; R Core Team, Vienna, Austria). Diagnostic performance data for AD testing (both ELISA and lateral flow assays) were extracted from each study, including sensitivity and specificity. Confidence intervals (95 % CI) were included when provided directly in the study.
Meta-analyses of sensitivity and specificity were performed separately for the AD-ELISA and AD-LF tests. Forest plots were generated using R software. Each subgroup included pooled sensitivity and specificity estimates, accounting for expected heterogeneity among studies. Heterogeneity describes the degree of variability in diagnostic performance estimates (sensitivity and specificity) across studies and was quantified using the I2 statistic. Values of 0 % indicate no observed heterogeneity, whereas increasing values reflect greater variability between study results. Only studies that included 95 % CI for sensitivity and specificity were included in the pooled analyses and forest plots. Median values for sensitivity and specificity were calculated across all studies.
3.1 Study selection and characteristics
A total of 51 studies were included in analysis (Table 1). Of these, 22 utilized the quantitative AD-ELISA test, 32 utilized the qualitative AD-LF test, and 2 did not specify the test type. Only 3 studies evaluated both the ELISA and AD-LF tests. In total, 723 patients with PJI were identified in the AD-ELISA group, and 765 patients with PJI were identified in the AD-LF group. Geographically, the studies included in the analysis originated from 15 countries. The majority were conducted in the United States (15) and Germany (10), followed by Italy (4), Austria (4), China (3), the Netherlands (3), Brazil (2), France (3), and Belgium (2); 1 study each came from Taiwan, Japan, Singapore, Türkiye, and Switzerland. Regarding study design, 39 studies were observational clinical studies, 8 studies were prospective, 3 were retrospective, and 1 was a clinical trial.
For the ELISA test, the reported cutoff ranged from 4.8 to 35.4 µg mL−1, although 5 studies did not disclose a specific threshold. In contrast, the AD-LF test is a qualitative test and therefore does not involve a defined numerical cutoff (Table 1).
Table 1Summary of articles included in the analysis.1
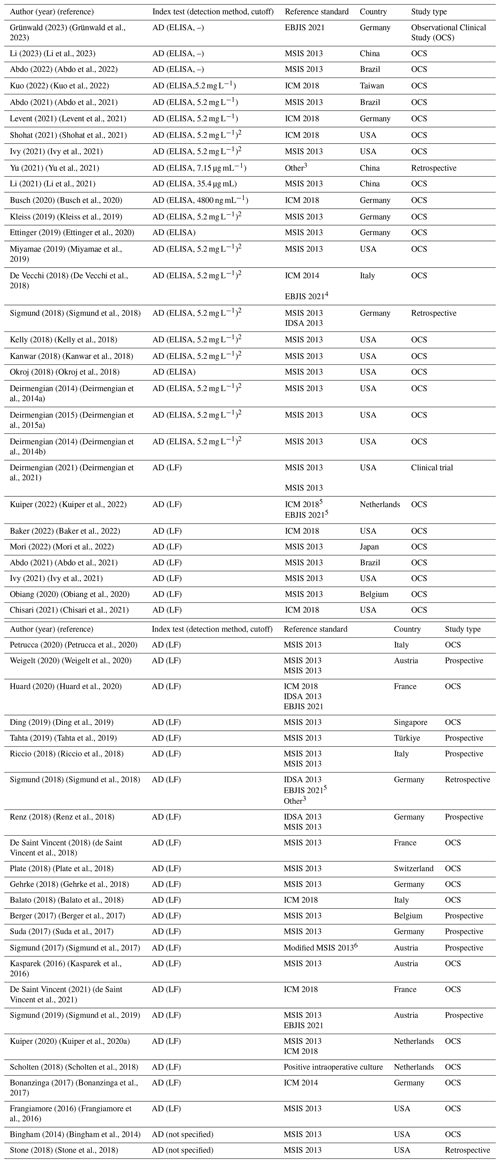
1 For reporting the median sensitivity and specificity in the summary report, in those studies where different criteria were evaluated, we used the results for the most recent criteria. 2 Providing results as a semiquantitative signal-to-cutoff ratio of 1.0. 3 Criteria: (1) purulence around the prosthesis or sinus tract, (2) increased synovial fluid leukocyte count, (3) positive histopathology, or (4) significant microbial growth in the synovial fluid, periprosthetic tissue, or sonicated culture. 4 Corresponds to the working draft that was finally published as EBJIS 2021 criteria. 5 Regarding inconclusive (ICM 2018) and likely (EBJIS 2021) as infected cases. 6 One of the following major criteria was present: a sinus track communicating with the joint, two positive conventional cultures with phenotypically identical microorganisms, or three minor criteria being met (elevated serum CRP levels, one positive microbiological culture, and positive histological analysis of periprosthetic tissue).
3.2 Diagnostic accuracy
3.2.1 Alpha-defensin ELISA assay
A total of 22 studies evaluated the diagnostic accuracy of the AD-ELISA test for the diagnosis of PJI, reporting a wide range of sensitivities and specificities (Table 2). Sensitivity values range from 0.5 (Sigmund et al., 2018) to 1.00 (Okroj et al., 2018; Deirmengian et al., 2015a, 2014b). Specificity ranged from 0.68 (Okroj et al., 2018) to 1.00 in multiple studies (Abdo et al., 2021; Deirmengian et al., 2014a, b, 2015b; Li et al., 2023, 2021; Miyamae et al., 2019). The median sensitivity and specificity were 0.8635 and 0.9675, respectively. When stratified by reference standard, the median sensitivity and specificity were 0.685 and 0.985 for EBJIS 2021, 0.927 and 0.963 for MSIS 2013, and 0.844 and 0.931 for ICM 2018.
Table 2Sensitivity and specificity of the alpha-defensin ELISA test for the diagnosis of periprosthetic joint infection across included studies, stratified by reference standard1.
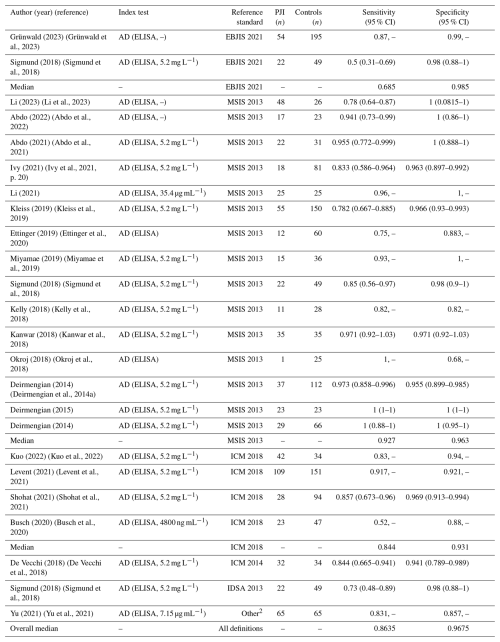
1 For reporting the median sensitivity and specificity in the summary report, in those studies where different criteria were evaluated, we used the results for the most recent criteria. 2 Criteria: (1) purulence around the prosthesis or sinus tract, (2) increased synovial fluid leukocyte count, (3) positive histopathology, or (4) significant microbial growth in the synovial fluid, periprosthetic tissue, or sonicated culture.
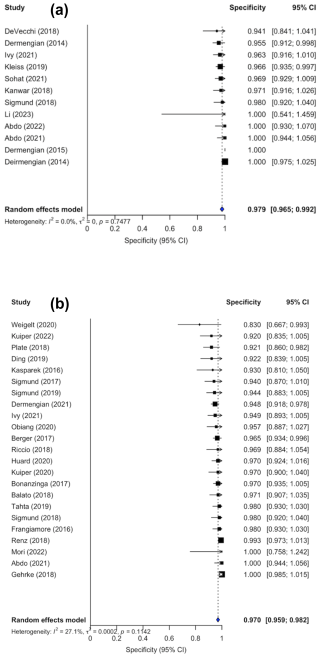
Confidence intervals were inconsistently reported; 10 out of the 22 studies did not include confidence interval in their analysis. Forest plots illustrating sensitivity (Fig. 2a) and specificity (Fig. 3a) were created exclusively from studies that included confidence interval data. The pooled sensitivity and specificity of the AD-ELISA assay was 0.878 (95 % CI: 0.812 to 0.943) and 0.979 (95 % CI: 0.965 to 0.992), respectively. Heterogeneity among studies for AD-ELISA assay was 78.2 % for sensitivity and 0 % for specificity.
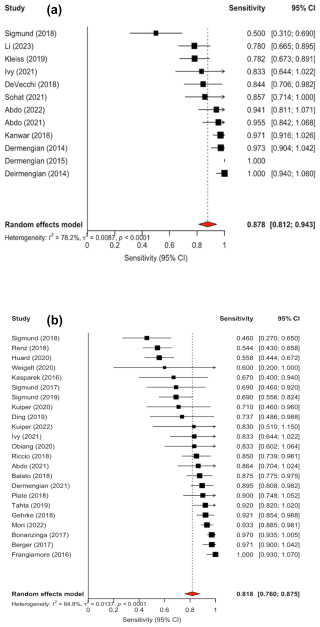
Figure 2(a) Forest plot of sensitivity for alpha-defensin ELISA assay in diagnosing periprosthetic joint infection. (b) Forest plot of sensitivity for alpha-defensin lateral flow assay in diagnosing periprosthetic joint infection.
3.2.2 Alpha-defensin lateral flow assay
The diagnostic accuracy of the AD-LF assay was evaluated across 30 studies (Table 3). Sensitivity and specificity values varied across studies, ranging from 0.2 (Scholten et al., 2018) to 1.00 (Frangiamore et al., 2016) and 0.824 (Suda et al., 2017) to 1.0, respectively (Abdo et al., 2021; Mori et al., 2022; Chisari et al., 2021; Gehrke et al., 2018; Scholten et al., 2018). The median sensitivity and specificity of the AD-LF was 0.8415 and 0.967, respectively. When stratified by reference standard, the median sensitivity and specificity were 0.514 and 0.975 for EBJIS 2021, 0.869 and 0.958 for MSIS 2013, 0.878 and 0.971 for ICM 2018, 0.673 and 0.955 for IDSA 2013, and 0.372 and 0.997 for studies using other criteria.
Confidence intervals were reported in the analysis of 23 out of the 30 studies. Forest plots for sensitivity (Fig. 2b) and specificity (Fig. 3b) were generated from the 23 studies with reported confidence intervals. The pooled sensitivity and specificity of the AD-LF assay were 0.818 (95 % CI: 0.760 to 0.875) and 0.970 (95 % CI: 0.959 to 0.982), respectively. Heterogeneity among studies for the AD-LF assay was 84.8 % for sensitivity and 27.1 % for specificity.
Table 3Sensitivity and specificity of alpha-defensin lateral flow test for the diagnosis of periprosthetic joint infection across included studies, stratified by reference standard.*
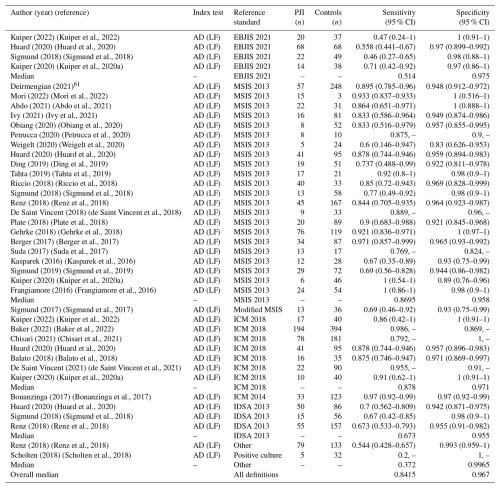
* For reporting the median sensitivity and specificity in the summary report, in those studies where different criteria were evaluated, we used the results for the most recent criteria.
3.2.3 Synovial fluid biomarkers (SFBs)
Synovial fluid biomarkers (SFBs) reflect the presence or activity of neutrophils. This analysis evaluated studies that compared the diagnostic performance of SFBs (AD-ELISA and AD-LF) against the standard synovial fluid tests of leukocyte count (LC) and percentage of polymorphonuclear neutrophils (PMN %). To prevent incorporation bias, only studies that excluded LC and PMN % from their PJI diagnostic criteria were included in the sub-analysis.
3.2.4 AD-ELISA
A total of 6 studies met the criteria for the AD-ELISA cohort comparison. The median sensitivity of AD-ELISA, LC, and PMN was 0.844 (95 % CI: 0.78 to 0.93), 0.872 (95 % CI: 0.74 to 0.93), and 0.881 (95 % CI: 0.796 to 0.94), respectively. The median specificity of AD-ELISA, LC, and PMN was 0.941 (95 % CI: 0.921 to 1), 0.912 (95 % CI: 0.71 to 1), and 0.84 (95 % CI: 0.682 to 0.841), respectively (Table 4).
Table 4Comparison of AD-ELISA, synovial fluid leukocyte count, and PMN % for PJI diagnosis.
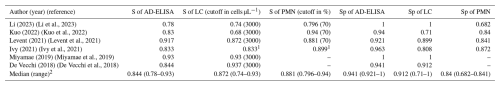
S, sensitivity. Sp, specificity. AD-LF, alpha-defensin lateral flow. LC, leukocyte count. PMN, polymorphonuclear neutrophils. %, percentage. 1 The cutoff for the LC was 3000 cells µL−1 in total hip arthroplasties (THAs) and 1700 cells µL−1 in total knee arthroplasties (TKAs). The cutoff for PMN was 80 % in THAs and 65 % in TKAs. 2 Considering only articles using an LC cutoff of 3000 cells µL−1 and a PMN of 70 %. Consideration: using an LC cutoff of 3000 cells µL−1 and a PMN of 70 %, AD-ELISA showed similar sensitivity and slightly higher specificity compared to both LC and PMN %.
3.2.5 AD-LF
A total of 11 studies met the criteria for the AD-LF cohort. The median sensitivity of AD-LF, LC, and PMN was 0.85 (95 % CI: 0.52 to 0.971), 0.864 (95 % CI: 0.75 to 0.902), and 0.842 (95 % CI: 0.75 to 0.895), respectively. The median specificity of AD-LF, LC, and PMN was 0.969 (95 % CI: 0.922 to 1), 0.94 (5 % CI: 0.764 to 0.989), and 0.949 (95 % CI: 0.903 to 1), respectively (Table 5).
Table 5Comparison of AD-LF, synovial fluid leukocyte count, and PMN % for PJI diagnosis.
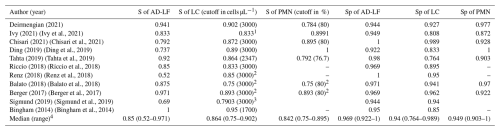
S, sensitivity. Sp, specificity. AD-LF, alpha-defensin lateral flow. LC, leukocyte count. PMN, polymorphonuclear neutrophils. %, percentage. 1 The cutoff for the LC was 3000 cells µL−1 in total hip arthroplasties (THAs) and 1700 cells µL−1 in total knee arthroplasties (TKAs). The cutoff for PMN was 80 % in THAs and 65 % in TKAs. 2 For the analysis, we have included the cohort of late PJI (Renz) or chronic PJI (Balato and Berger). 3 The cutoff for LC was > 10 000 cells µL−1 for acute PJI and > 3000 cells µL−1 for chronic PJI. Data in acute and chronic PJI were aggregated, so it is not possible to split the information. 4 Considering only articles using LC cutoff of 3000 cells µL−1 and a PMN of 80 %. Consideration: using an LC cutoff of 3000 cells µL−1 and a PMN of 80 %, AD-LF showed similar sensitivity and specificity compared to both LC and PMN %.
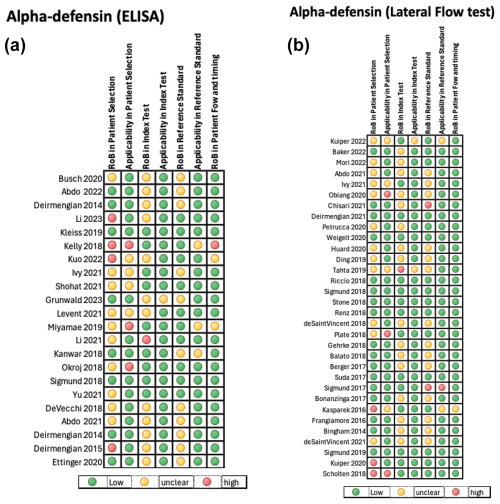
3.3 Quality assessment
The quality of the included studies assessing the diagnostic accuracy of the AD-ELISA and LF assay was evaluated using the QUADAS-2 tool. A pooled analysis of each domain is demonstrated in Fig. 4a and b. The AD-ELISA group had a mean of 66 % low risk of bias across all domains, whereas the AD-LF group had a mean of 74 %. Figure 5a and b demonstrate the risk of bias of each individual study included in analysis.
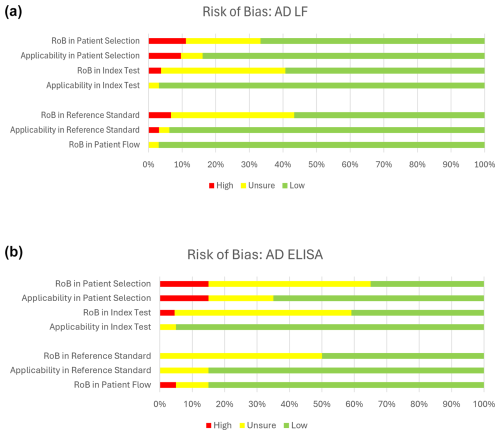
Figure 4(a) Risk of bias for alpha-defensin lateral flow assay (AD-LF). Based on QUADAS-2 assessment. (b) Risk of bias for alpha-defensin ELISA assay (AD-LF). Based on QUADAS-2 assessment.
This systematic review and meta-analysis included 51 studies evaluating the diagnostic accuracy of both the laboratory-based AD-ELISA and the quantitative AD-LF in detecting PJI. Both assays demonstrated excellent specificity, with pooled values of 97.9 % for ELISA and 97.0 % for LF, confirming their reliability as rule-in tests. However, ELISA exhibited higher pooled sensitivity than LF (87.8 % vs. 81.8 %), a difference that likely reflects the intrinsic advantages of its quantitative laboratory design. By measuring alpha-defensin concentration through an enzymatic reaction, ELISA can detect low biomarker levels that may be missed by the qualitative, threshold-based LF assay. Consequently, ELISA demonstrates superior sensitivity and may provide greater reproducibility across a broader range of clinical presentations, including low-grade and indolent infections, while maintaining comparable specificity. Across included studies, heterogeneity was minimal for specificity but substantial for sensitivity, indicating consistent performance in confirming infection but variable reliability in ruling it out. A targeted sub-analysis of studies excluding LC and PMN % from the diagnostic reference standard demonstrated that both AD-ELISA and AD-LF exhibited sensitivity comparable to LC and PMN % but with consistently higher specificity.
There have been several prior systematic reviews and meta-analyses on AD in the diagnosis of PJI (Ahmad et al., 2018; Balato et al., 2018; Carli et al., 2019; Eriksson et al., 2018; Lee et al., 2017; Li et al., 2017; Marson et al., 2018; Saleh et al., 2017; Suen et al., 2018; Vale et al., 2023; Wyatt et al., 2016; Xie et al., 2017; Yuan et al., 2017), with the most recent review published in 2023 (Vale et al., 2023). However, many of these studies employed narrower inclusion criteria, or the meta-analyses have become outdated given the growing body of recent literature on AD in PJI diagnosis. For example, Kuiper et al. (2020b) included only prospective studies in their analysis, while Marson et al. (2018) and Balato et al. (2020) limited inclusion to studies using the MSIS as the diagnostic reference standard. Vale et al. (2023) comprehensively investigated synovial fluid biomarkers across various PJI criteria, reporting favorable diagnostic accuracy for AD consistent with the present study. The present study differs from the previous literature in a few important ways. The present study performed separate pooled analyses for AD-ELISA and AD-LF, allowing more granular comparisons. Additionally, this study incorporated both prospective and retrospective studies and accepted a broader range of validated reference standards, including MSIS 2013, ICM 2018, EBJIS 2021, and IDSA guidelines. Notably, conducting a targeted sub-analysis limited studies that excluded leukocyte count and PMN % from their diagnostic criteria, thereby minimizing incorporation bias and enabling a more direct comparison between AD testing and traditional synovial fluid markers. The methodological breadth, incorporation of the most recent evidence, and strict requirement for accepted gold-standard diagnostic criteria for PJI position our review as one of the most comprehensive evaluations on AD assay performance to date.
A definitive gold standard for diagnosing PJI has yet to be established. Among the available diagnostic tools, synovial fluid analysis has demonstrated the greatest promise for identifying PJI cases with accuracy, outperforming serum markers (Goud et al., 2022). While commonly used, traditional inflammatory markers including white blood cell count (WBC), synovial WBC count, PMN %, and CRP are limited by variable sensitivity, false negatives, and delays in turnaround time (Kheir et al., 2018; Quinlan and Jennings, 2023). Consequently, a growing number of synovial fluid markers have been investigated beyond AD, with notable focus on calprotectin (Peng et al., 2022), interleukin-6 (IL-6) (Li et al., 2022), and leukocyte esterase (Vale et al., 2023), among others. Despite promising early data, many of these alternative biomarkers remain under evaluation, with limited standardization and inconsistent incorporation into consensus guidelines. Further comparative studies are needed to determine the optimal biomarker or combination of markers that can reliably distinguish PJI from aseptic failure across diverse clinical scenarios in TJA.
Quality was assessed using the QUADAS-2 tool, which evaluates risk of bias across four key domains (patient selection, index test, reference standard, and patient flow and timing), along with applicability concerns for each of the first three domains. Overall, the studies included demonstrated low to moderate risk of bias, with the most frequent concerns related to the index test and patient selection domains. Several studies lacked blinding or did not clearly pre-specify thresholds for the index test, which may overestimate diagnostic performance. Nonetheless, the majority adhered to established diagnostic criteria and provided sufficient methodological detail to support inclusion. These findings highlight the importance of cautious interpretation of pooled estimates, especially in the context of heterogeneous study designs and potential incorporation bias.
There are several limitations to this study that should be acknowledged. This meta-analysis and systematic review included significant heterogeneity across the studies included in terms of patient populations, diagnostic criteria, and surgical settings, which may contribute to variability in the reported values. Additionally, confidence intervals were not consistently reported in the included studies, limiting the precision of the pooled estimates of sensitivity and specificity. There is also the potential for publication bias, as studies with favorable results may be more likely to be published. The diagnostic performance of AD may be influenced by implant location due to all total joint arthroplasty being included, chronicity of infection, or underlying inflammatory conditions that were not specifically stratified in our analysis. Finally, a large proportion of the studies included were observational, lacking standardized blinding and clearly defined index test thresholds, introducing the potential for both performance and detection bias.
Both AD assays demonstrate high specificity in diagnosing PJI, but AD-ELISA offers superior sensitivity compared to AD-LF and traditional synovial markers. Given variability in the underlying diagnostic criteria for PJI, these results should be interpreted within the context of differing reference standards. These findings support the continued use of AD-ELISA as a valid diagnostic modality.
All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria). Analysis scripts and output files are available from the corresponding author upon reasonable request.
The data used in this study are not publicly available but can be obtained from the corresponding author upon reasonable request.
BP: writing (original draft), investigation, conceptualization. DD: writing (review and editing), investigation, methodology, conceptualization. AS: writing (review and editing), visualization, methodology, investigation, formal analysis. AM: writing (review and editing), supervision, project administration, methodology. TS: Writing (review and editing), validation, data curation, supervision, methodology, investigation.
At least one of the (co-)authors is a member of the editorial board of Journal of Bone and Joint Infection. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
No institutional review board approval was needed due to the review nature of this article.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This article was put together by the Synovial Biomarker Workgroup for the Unified PJI Definition.
This paper was edited by Derek Amanatullah and reviewed by two anonymous referees.
Abdo, R. C. T., Gobbi, R. G., Leite, C. B. G., Pasoto, S. G., Leon, E. P., Lima, A. L. L. M., Bonfa, E., Pécora, J. R., and Demange, M. K.: Performance of alpha-defensin lateral flow test after synovial fluid centrifugation for diagnosis of periprosthetic knee infection, World J. Orthop., 12, 565–574, https://doi.org/10.5312/wjo.v12.i8.565, 2021.
Abdo, R. C. T., Gobbi, R. G., Leite, C. B. G., Pasoto, S. G., Leon, E. P., Lima, A. L. L. M., Bonfa, E., Pécora, J. R., and Demange, M. K.: Quantitative alpha-defensin testing: Is synovial fluid dilution important?, World J. Orthop., 13, 760–767, https://doi.org/10.5312/wjo.v13.i8.760, 2022.
Ahmad, S. S., Hirschmann, M. T., Becker, R., Shaker, A., Ateschrang, A., Keel, M. J. B., Albers, C. E., Buetikofer, L., Maqungo, S., Stöckle, U., and Kohl, S.: A meta-analysis of synovial biomarkers in periprosthetic joint infection: Synovasure™ is less effective than the ELISA-based alpha-defensin test, Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA, 26, 3039–3047, https://doi.org/10.1007/s00167-018-4904-8, 2018.
Baker, C. M., Goh, G. S., Tarabichi, S., Shohat, N., and Parvizi, J.: Synovial C-Reactive Protein is a Useful Adjunct for Diagnosis of Periprosthetic Joint Infection, J. Arthroplasty, 37, 2437-2443.e1, https://doi.org/10.1016/j.arth.2022.06.016, 2022.
Balato, G., Franceschini, V., Ascione, T., Lamberti, A., D'Amato, M., Ensini, A., and Baldini, A.: High performance of α-defensin lateral flow assay (Synovasure) in the diagnosis of chronic knee prosthetic infections, Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA, 26, 1717–1722, https://doi.org/10.1007/s00167-017-4745-x, 2018.
Balato, G., de Matteo, V., Ascione, T., Di Donato, S. L., De Franco, C., Smeraglia, F., Baldini, A., and Mariconda, M.: Laboratory-based versus qualitative assessment of α-defensin in periprosthetic hip and knee infections: a systematic review and meta-analysis, Arch. Orthop. Trauma Surg., 140, 293–301, https://doi.org/10.1007/s00402-019-03232-5, 2020.
Berger, P., Van Cauter, M., Driesen, R., Neyt, J., Cornu, O., and Bellemans, J.: Diagnosis of prosthetic joint infection with alpha-defensin using a lateral flow device: a multicentre study, Bone Jt. J., 99-B, 1176–1182, https://doi.org/10.1302/0301-620X.99B9.BJJ-2016-1345.R2, 2017.
Bingham, J., Clarke, H., Spangehl, M., Schwartz, A., Beauchamp, C., and Goldberg, B.: The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty, Clin. Orthop., 472, 4006–4009, https://doi.org/10.1007/s11999-014-3900-7, 2014.
Bonanzinga, T., Zahar, A., Dütsch, M., Lausmann, C., Kendoff, D., and Gehrke, T.: How Reliable Is the Alpha-defensin Immunoassay Test for Diagnosing Periprosthetic Joint Infection? A Prospective Study, Clin. Orthop., 475, 408–415, https://doi.org/10.1007/s11999-016-4906-0, 2017.
Busch, A., Jäger, M., Engler, H., Haversath, M., Bielefeld, C., Landgraeber, S., and Wegner, A.: Is Procalcitonin (PCT) a reliable biomarker for preoperative diagnosing of low grade periprosthetic joint infection? A prospective study, BMC Musculoskelet. Disord., 21, 257, https://doi.org/10.1186/s12891-020-03266-6, 2020.
Carli, A. V., Abdelbary, H., Ahmadzai, N., Cheng, W., Shea, B., Hutton, B., Sniderman, J., Philip Sanders, B. S., Esmaeilisaraji, L., Skidmore, B., Gauthier-Kwan, O. Y., Bunting, A. C., Gauthier, P., Crnic, A., Logishetty, K., Moher, D., Fergusson, D., and Beaulé, P. E.: Diagnostic Accuracy of Serum, Synovial, and Tissue Testing for Chronic Periprosthetic Joint Infection After Hip and Knee Replacements: A Systematic Review, J. Bone Joint Surg. Am., 101, 635–649, https://doi.org/10.2106/JBJS.18.00632, 2019.
Chisari, E., Yacovelli, S., Goswami, K., Shohat, N., Woloszyn, P., and Parvizi, J.: Leukocyte Esterase Versus ICM 2018 Criteria in the Diagnosis of Periprosthetic Joint Infection, J. Arthroplasty, 36, 2942-2945.e1, https://doi.org/10.1016/j.arth.2021.03.006, 2021.
Deirmengian, C., Lonner, J. H., and Booth, R. E.: The Mark Coventry Award: white blood cell gene expression: a new approach toward the study and diagnosis of infection, Clin. Orthop., 440, 38–44, https://doi.org/10.1097/01.blo.0000185756.17401.32, 2005.
Deirmengian, C., Kardos, K., Kilmartin, P., Cameron, A., Schiller, K., and Parvizi, J.: Combined measurement of synovial fluid α-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection, J. Bone Joint Surg. Am., 96, 1439–1445, https://doi.org/10.2106/JBJS.M.01316, 2014a.
Deirmengian, C., Kardos, K., Kilmartin, P., Cameron, A., Schiller, K., and Parvizi, J.: Diagnosing periprosthetic joint infection: has the era of the biomarker arrived?, Clin. Orthop., 472, 3254–3262, https://doi.org/10.1007/s11999-014-3543-8, 2014b.
Deirmengian, C., Kardos, K., Kilmartin, P., Cameron, A., Schiller, K., Booth, R. E. J., and Parvizi, J.: The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip, Clin. Orthop., 473, 198–203, https://doi.org/10.1007/s11999-014-3722-7, 2015a.
Deirmengian, C., Kardos, K., Kilmartin, P., Cameron, A., Schiller, K., Booth, R. E. J., and Parvizi, J.: The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip, Clin. Orthop., 473, 198–203, https://doi.org/10.1007/s11999-014-3722-7, 2015b.
Deirmengian, C., Madigan, J., Kallur Mallikarjuna, S., Conway, J., Higuera, C., and Patel, R.: Validation of the Alpha Defensin Lateral Flow Test for Periprosthetic Joint Infection, J. Bone Joint Surg. Am., 103, 115–122, https://doi.org/10.2106/JBJS.20.00749, 2021.
de Saint Vincent, B., Migaud, H., Senneville, E., Loiez, C., Pasquier, G., Girard, J., and Putman, S.: Diagnostic accuracy of the alpha defensin lateral flow device (Synovasure) for periprosthetic infections in microbiologically complex situations: A study of 42 cases in a French referral centre, Orthop. Traumatol. Surg. Res. OTSR, 104, 427–431, https://doi.org/10.1016/j.otsr.2018.01.018, 2018.
de Saint Vincent, B., Martinot, P., Pascal, A., Senneville, E., Loiez, C., Pasquier, G., Girard, J., Putman, S., and Migaud, H.: Does the alpha-defensin lateral flow test conserve its diagnostic properties in a larger population of chronic complex periprosthetic infections? Enlargement to 112 tests, from 42 tests in a preliminary study, in a reference center, Orthop. Traumatol. Surg. Res., 107, https://doi.org/10.1016/j.otsr.2021.102912, 2021.
De Vecchi, E., Romanò, C. L., De Grandi, R., Cappelletti, L., Villa, F., and Drago, L.: Alpha defensin, leukocyte esterase, C-reactive protein, and leukocyte count in synovial fluid for pre-operative diagnosis of periprosthetic infection, Int. J. Immunopathol. Pharmacol., 32, 2058738418806072, https://doi.org/10.1177/2058738418806072, 2018.
Ding, B. T., Tan, K. G., Kau, C. Y., Chan, H. Y. H., and Mohd Fadil, M. F. B.: Accuracy of the α-defensin lateral flow assay for diagnosing periprosthetic joint infection in Asians, J. Orthop. Surg. Hong Kong, 27, 2309499019828459, https://doi.org/10.1177/2309499019828459, 2019.
Eriksson, H. K., Nordström, J., Gabrysch, K., Hailer, N. P., and Lazarinis, S.: Does the Alpha-defensin Immunoassay or the Lateral Flow Test Have Better Diagnostic Value for Periprosthetic Joint Infection? A Meta-analysis, Clin. Orthop., 476, 1065–1072, https://doi.org/10.1007/s11999.0000000000000244, 2018.
Ettinger, M., Savov, P., Calliess, T., Windhagen, H., Lichtinghagen, R., Lukasz, A., and Omar, M.: Improved diagnostic accuracy with the classification tree method for diagnosing low-grade periprosthetic joint infections by quantitative measurement of synovial fluid alpha-defensin and C-reactive protein, Int. Orthop., 44, 31–38, https://doi.org/10.1007/s00264-019-04338-6, 2020.
Frangiamore, S. J., Gajewski, N. D., Saleh, A., Farias-Kovac, M., Barsoum, W. K., and Higuera, C. A.: α-Defensin Accuracy to Diagnose Periprosthetic Joint Infection-Best Available Test?, J. Arthroplasty, 31, 456–460, https://doi.org/10.1016/j.arth.2015.09.035, 2016.
Gehrke, T., Lausmann, C., Citak, M., Bonanzinga, T., Frommelt, L., and Zahar, A.: The Accuracy of the Alpha Defensin Lateral Flow Device for Diagnosis of Periprosthetic Joint Infection: Comparison with a Gold Standard, J. Bone Joint Surg. Am., 100, 42–48, https://doi.org/10.2106/JBJS.16.01522, 2018.
Goud, A., Nützinger, D., van der Bij, A., Jenniskens, K., Groenewold, J., de Gast, A., and Bekkers, J. E. J.: Synovial-Based Tests Outperform Serum Markers to Rule Out Infection in Total Knee Arthroplasty and Total Hip Arthroplasty: A Systematic Review and Meta-Analysis, J. Arthroplasty, 37, 802-808.e5, https://doi.org/10.1016/j.arth.2021.12.020, 2022.
Grünwald, L., Schmidutz, F., Döttger, P., Erne, F., Schreiner, A. J., and Hemmann, P.: Leukocyte esterase and alpha-defensin in periprosthetic joint infection: predictive quality and correlation in a prospective study, Int. Orthop., 47, 2663–2668, https://doi.org/10.1007/s00264-023-05914-7, 2023.
Huard, M., Detrembleur, C., Poilvache, H., Pastor Y Geels, I., Van Cauter, M., Driesen, R., Yombi, J.-C., Neyt, J., and Cornu, O.: Alpha Defensin: A Diagnostic Accuracy Depending on the Infection Definition Used, J. Arthroplasty, 35, 1355–1360, https://doi.org/10.1016/j.arth.2019.12.010, 2020.
Hubert, J., Ritter, J., Krüger, L., Simon, A., Beil, F. T., Jandl, N. M., and Rolvien, T.: Are Synovial Inflammatory Markers Increased in Patients Who Have Aseptic Total Hip Arthroplasty Dislocation Indicated for Revision?, J. Arthroplasty, 39, 787-794.e1, https://doi.org/10.1016/j.arth.2023.08.054, 2024.
Ivy, M. I., Sharma, K., Greenwood-Quaintance, K. E., Tande, A. J., Osmon, D. R., Berbari, E. F., Mandrekar, J., Beauchamp, C. P., Hanssen, A. D., Abdel, M. P., Lewallen, D. G., Perry, K., Block, D. R., Snyder, M. R., and Patel, R.: Synovial fluid α defensin has comparable accuracy to synovial fluid white blood cell count and polymorphonuclear percentage for periprosthetic joint infection diagnosis, Bone Jt. J., 103-B, 1119–1126, https://doi.org/10.1302/0301-620X.103B6.BJJ-2020-1741.R1, 2021.
Kanwar, S., Al-Mansoori, A. A., Chand, M. R., Villa, J. M., Suarez, J. C., and Patel, P. D.: What Is the Optimal Criteria to Use for Detecting Periprosthetic Joint Infections Before Total Joint Arthroplasty?, J. Arthroplasty, 33, S201–S204, https://doi.org/10.1016/j.arth.2018.02.072, 2018.
Kasparek, M. F., Kasparek, M., Boettner, F., Faschingbauer, M., Hahne, J., and Dominkus, M.: Intraoperative Diagnosis of Periprosthetic Joint Infection Using a Novel Alpha-Defensin Lateral Flow Assay, J. Arthroplasty, 31, 2871–2874, https://doi.org/10.1016/j.arth.2016.05.033, 2016.
Kelly, M. P., Darrith, B., Hannon, C. P., Nam, D., Courtney, P. M., and Della Valle, C. J.: Synovial Fluid Alpha-Defensin Is an Adjunctive Tool in the Equivocal Diagnosis of Periprosthetic Joint Infection, J. Arthroplasty, 33, 3537–3540, https://doi.org/10.1016/j.arth.2018.06.026, 2018.
Kheir, M. M., Tan, T. L., Shohat, N., Foltz, C., and Parvizi, J.: Routine Diagnostic Tests for Periprosthetic Joint Infection Demonstrate a High False-Negative Rate and Are Influenced by the Infecting Organism, JBJS, 100, 2057, https://doi.org/10.2106/JBJS.17.01429, 2018.
Kleiss, S., Jandl, N. M., Novo de Oliveira, A., Rüther, W., and Niemeier, A.: Diagnostic accuracy of alpha-defensin enzyme-linked immunosorbent assay in the clinical evaluation of painful hip and knee arthroplasty with possible prosthetic joint infection: a prospective study of 202 cases, Bone Jt. J., 101-B, 970–977, https://doi.org/10.1302/0301-620X.101B8.BJJ-2018-1390.R2, 2019.
Kuiper, J. W., Pander, P., and Vos, S. J.: Good accuracy of the alpha-defensin lateral flow test for hip periprosthetic joint infection: A pilot study in a retrospective cohort of 52 patients, World J. Orthop., 11, 36–46, https://doi.org/10.5312/wjo.v11.i1.36, 2020a.
Kuiper, J. W. P., Verberne, S. J., Vos, S. J., and van Egmond, P. W.: Does the Alpha Defensin ELISA Test Perform Better Than the Alpha Defensin Lateral Flow Test for PJI Diagnosis? A Systematic Review and Meta-analysis of Prospective Studies, Clin. Orthop., 478, 1333–1344, https://doi.org/10.1097/CORR.0000000000001225, 2020b.
Kuiper, J. W. P., Verberne, S. J., van Egmond, P. W., Slot, K., Temmerman, O. P. P., and Vos, C. J.: Are Accuracy Studies for Periprosthetic Joint Infection Diagnosis Inherently Flawed? And What to Do with Schrödinger's Hips? A Prospective Analysis of the Alpha Defensin Lateral-Flow Test in Chronic Painful Hip Arthroplasties, Hip Pelvis, 34, 236–244, https://doi.org/10.5371/hp.2022.34.4.236, 2022.
Kuo, F.-C., Lin, P.-C., Yen, S.-H., Tan, T. L., Wu, C.-T., and Wang, J.-W.: Which Minor Criteria is the Most Accurate Predictor for the Diagnosis of Hip and Knee Periprosthetic Joint Infection in the Asian Population?, J. Arthroplasty, 37, 2076–2081, https://doi.org/10.1016/j.arth.2022.05.002, 2022.
Lee, Y. S., Koo, K.-H., Kim, H. J., Tian, S., Kim, T.-Y., Maltenfort, M. G., and Chen, A. F.: Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis, J. Bone Joint Surg. Am., 99, 2077–2084, https://doi.org/10.2106/JBJS.17.00123, 2017.
Lehrer, R. I. and Ganz, T.: Defensins: endogenous antibiotic peptides from human leukocytes, Ciba Found. Symp., 171, 276–290, discussion 290-293, https://doi.org/10.1002/9780470514344.ch16, 1992.
Levent, A., Neufeld, M. E., Piakong, P., Lausmann, C., Gehrke, T., and Citak, M.: Which International Consensus Meeting Preoperative Minor Criteria is the Most Accurate Marker for the Diagnosis of Periprosthetic Joint Infection in Hip and Knee Arthroplasty?, J. Arthroplasty, 36, 3728–3733, https://doi.org/10.1016/j.arth.2021.06.030, 2021.
Li, B., Chen, F., Liu, Y., and Xu, G.: Synovial Fluid α-Defensin as a Biomarker for Peri-Prosthetic Joint Infection: A Systematic Review and Meta-Analysis, Surg. Infect., 18, 702–710, https://doi.org/10.1089/sur.2017.006, 2017.
Li, H., Li, R., Erlong, N., Chai, W., Hao, L., Xu, C., Fu, J., Chen, J., and Zhu, F.: It can be unnecessary to combine common synovial fluid analysis and alpha-defensin tests for periprosthetic joint infection diagnosis, BMC Musculoskelet. Disord., 24, 529, https://doi.org/10.1186/s12891-023-06594-5, 2023.
Li, J., Zhou, Q., and Deng, B.: Serum versus synovial fluid interleukin-6 for periprosthetic joint infection diagnosis: a systematic review and meta-analysis of 30 diagnostic test accuracy studies, J. Orthop. Surg., 17, 564, https://doi.org/10.1186/s13018-022-03458-x, 2022.
Li, R., Li, X., Ni, M., Fu, J., Xu, C., Chai, W., and Chen, J.-Y.: What is the performance of novel synovial biomarkers for detecting periprosthetic joint infection in the presence of inflammatory joint disease?, Bone Jt. J., 103-B, 32–38, https://doi.org/10.1302/0301-620X.103B1.BJJ-2019-1479.R3, 2021.
Marson, B. A., Deshmukh, S. R., Grindlay, D. J. C., and Scammell, B. E.: Alpha-defensin and the Synovasure lateral flow device for the diagnosis of prosthetic joint infection: a systematic review and meta-analysis., Bone Jt. J., 100-B, 703–711, https://doi.org/10.1302/0301-620X.100B6.BJJ-2017-1563.R1, 2018.
McNally, M., Sousa, R., Wouthuyzen-Bakker, M., Chen, A. F., Soriano, A., Vogely, H. C., Clauss, M., Higuera, C. A., and Trebše, R.: The EBJIS definition of periprosthetic joint infection, Bone Jt. J., 103-B, 18–25, https://doi.org/10.1302/0301-620X.103B1.BJJ-2020-1381.R1, 2021.
Miyamae, Y., George, J., Klika, A. K., Barsoum, W. K., and Higuera, C. A.: Diagnostic Accuracy of the Alpha-Defensin Test for Periprosthetic Joint Infection in Patients With Inflammatory Diseases, J. Arthroplasty, 34, 1767–1771, https://doi.org/10.1016/j.arth.2019.04.020, 2019.
Mori, Y., Kanabuchi, R., Baba, K., Chiba, D., Kamimura, M., Mori, N., and Aizawa, T.: Evaluation of the usefulness of the Synovasure alpha-defensin lateral flow test kit for the diagnosis of periprosthetic joint infection in Japanese patients, J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc., 27, 935–938, https://doi.org/10.1016/j.jos.2022.05.001, 2022.
Obiang, A.-C., Laurent, F., Thayse, K., Rossi, C., and Collard, X.: Alpha-defensin for the intra-operative diagnosis of prosthetic joint infections, Acta Orthop. Belg., 86, 614–620, 2020.
Okroj, K. T., Calkins, T. E., Kayupov, E., Kheir, M. M., Bingham, J. S., Beauchamp, C. P., Parvizi, J., and Della Valle, C. J.: The Alpha-Defensin Test for Diagnosing Periprosthetic Joint Infection in the Setting of an Adverse Local Tissue Reaction Secondary to a Failed Metal-on-Metal Bearing or Corrosion at the Head-Neck Junction, J. Arthroplasty, 33, 1896–1898, https://doi.org/10.1016/j.arth.2018.01.007, 2018.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., Wilson, W. R., and Infectious Diseases Society of America: Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., 56, e1–e25, https://doi.org/10.1093/cid/cis803, 2013.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., McGuinness, L. A., Stewart, L. A., Thomas, J., Tricco, A. C., Welch, V. A., Whiting, P., and Moher, D.: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ, 372, n71, https://doi.org/10.1136/bmj.n71, 2021.
Parvizi, J. and Gehrke, T.: Definition of Periprosthetic Joint Infection, J. Arthroplasty, 29, 1331, https://doi.org/10.1016/j.arth.2014.03.009, 2014.
Parvizi, J., Tan, T. L., Goswami, K., Higuera, C., Della Valle, C., Chen, A. F., and Shohat, N.: The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria, J. Arthroplasty, 33, 1309-1314.e2, https://doi.org/10.1016/j.arth.2018.02.078, 2018.
Patel, R., Alijanipour, P., and Parvizi, J.: Advancements in Diagnosing Periprosthetic Joint Infections after Total Hip and Knee Arthroplasty, Open Orthop. J., 10, 654–661, https://doi.org/10.2174/1874325001610010654, 2016.
Peng, X., Zhang, H., Xin, P., Bai, G., Ge, Y., Cai, M., Wang, R., Fan, Y., and Pang, Z.: Synovial calprotectin for the diagnosis of periprosthetic joint infection: a diagnostic meta-analysis, J. Orthop. Surg., 17, 2, https://doi.org/10.1186/s13018-021-02746-2, 2022.
Petrucca, A., Santino, I., Gentile, G., Mazza, D., Viglietta, E., Iorio, R., Simmaco, M., Ferretti, A., and Borro, M.: Detection of α-defensin in synovial fluids by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry as an innovative and cost-effective assay for improved definition of periprosthetic joint infections, Rapid Commun. Mass Spectrom. RCM, 34, e8791, https://doi.org/10.1002/rcm.8791, 2020.
Plate, A., Stadler, L., Sutter, R., Anagnostopoulos, A., Frustaci, D., Zbinden, R., Fucentese, S. F., Zinkernagel, A. S., Zingg, P. O., and Achermann, Y.: Inflammatory disorders mimicking periprosthetic joint infections may result in false-positive α-defensin., Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis., 24, 1212.e1–1212.e6, https://doi.org/10.1016/j.cmi.2018.02.019, 2018.
Quinlan, N. D. and Jennings, J. M.: Joint aspiration for diagnosis of chronic periprosthetic joint infection: when, how, and what tests?, Arthroplasty, 5, https://doi.org/10.1186/s42836-023-00199-y, 2023.
Renz, N., Yermak, K., Perka, C., and Trampuz, A.: Alpha Defensin Lateral Flow Test for Diagnosis of Periprosthetic Joint Infection: Not a Screening but a Confirmatory Test, J. Bone Joint Surg. Am., 100, 742–750, https://doi.org/10.2106/JBJS.17.01005, 2018.
Riccio, G., Cavagnaro, L., Akkouche, W., Carrega, G., Felli, L., and Burastero, G.: Qualitative Alpha-defensin Versus The Main Available Tests For The Diagnosis Of Periprosthetic Joint Infection: Best Predictor Test?, J. Bone Joint Infect., 3, 156–164, https://doi.org/10.7150/jbji.26401, 2018.
Saleh, A., Ramanathan, D., Siqueira, M. B. P., Klika, A. K., Barsoum, W. K., and Rueda, C. A. H.: The Diagnostic Utility of Synovial Fluid Markers in Periprosthetic Joint Infection: A Systematic Review and Meta-analysis, J. Am. Acad. Orthop. Surg., 25, 763–772, https://doi.org/10.5435/JAAOS-D-16-00548, 2017.
Scholten, R., Visser, J., Van Susante, J., and Van Loon, C.: Low sensitivity of alpha-defensin (synovasure) test for intraoperative exclusion of prosthetic joint infection, HIP Int., 28, 87, https://doi.org/10.1177/11207000188011, 2018.
Shohat, N., Yacovelli, S., Chisari, E., Clarkson, S., Mann, D., and Parvizi, J.: Alpha-defensin does not provide additional benefit over leukocyte esterase in the diagnosis of periprosthetic joint infection, Expert Rev. Mol. Diagn., 21, 845–849, https://doi.org/10.1080/14737159.2021.1943364, 2021.
Sigmund, I. K., Holinka, J., Gamper, J., Staats, K., Böhler, C., Kubista, B., and Windhager, R.: Qualitative α-defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty, Bone Jt. J., 99-B, 66–72, https://doi.org/10.1302/0301-620X.99B1.BJJ-2016-0295.R1, 2017.
Sigmund, I. K., Yermak, K., Perka, C., Trampuz, A., and Renz, N.: Is the Enzyme-linked Immunosorbent Assay More Accurate Than the Lateral Flow Alpha Defensin Test for Diagnosing Periprosthetic Joint Infection?, Clin. Orthop., 476, 1645–1654, https://doi.org/10.1097/CORR.0000000000000336, 2018.
Sigmund, I. K., Holinka, J., Lang, S., Stenicka, S., Staats, K., Hobusch, G., Kubista, B., and Windhager, R.: A comparative study of intraoperative frozen section and alpha defensin lateral flow test in the diagnosis of periprosthetic joint infection, Acta Orthop., 90, 105–110, https://doi.org/10.1080/17453674.2019.1567153, 2019.
Slullitel, P. A., Oñativia, J. I., Buttaro, M. A., Sánchez, M. L., Comba, F., Zanotti, G., and Piccaluga, F.: State-of-the-art diagnosis and surgical treatment of acute peri-prosthetic joint infection following primary total hip arthroplasty, EFORT Open Rev., 3, 434–441, https://doi.org/10.1302/2058-5241.3.170032, 2018.
Stone, W. Z., Gray, C. F., Parvataneni, H. K., Al-Rashid, M., Vlasak, R. G., Horodyski, M., and Prieto, H. A.: Clinical Evaluation of Synovial Alpha Defensin and Synovial C-Reactive Protein in the Diagnosis of Periprosthetic Joint Infection, J. Bone Joint Surg. Am., 100, 1184–1190, https://doi.org/10.2106/JBJS.17.00556, 2018.
Suda, A. J., Tinelli, M., Beisemann, N. D., Weil, Y., Khoury, A., and Bischel, O. E.: Diagnosis of periprosthetic joint infection using alpha-defensin test or multiplex-PCR: ideal diagnostic test still not found, Int. Orthop., 41, 1307–1313, https://doi.org/10.1007/s00264-017-3412-7, 2017.
Suen, K., Keeka, M., Ailabouni, R., and Tran, P.: Synovasure “quick test” is not as accurate as the laboratory-based α-defensin immunoassay: a systematic review and meta-analysis, Bone Jt. J., 100-B, 66–72, https://doi.org/10.1302/0301-620X.100B1.BJJ-2017-0630.R1, 2018.
Tahta, M., Simsek, M. E., Isik, C., Akkaya, M., Gursoy, S., and Bozkurt, M.: Does inflammatory joint diseases affect the accuracy of infection biomarkers in patients with periprosthetic joint infections? A prospective comparative reliability study, J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc., 24, 286–289, https://doi.org/10.1016/j.jos.2018.08.022, 2019.
Vale, J. S., Castelo, F. S., Barros, B. S., Ribau, A. C., Carvalho, A. D., and Sousa, R. J. G.: Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection-A Systematic Review and Meta-Analysis of Their Diagnostic Accuracy According to Different Definitions, J. Arthroplasty, 38, 2731-2738.e3, https://doi.org/10.1016/j.arth.2023.06.017, 2023.
Weigelt, L., Plate, A., Stadler, L., Sutter, R., Frustaci, D., Zbinden, R., Zingg, P. O., Gerber, C., and Achermann, Y.: Alpha-defensin lateral flow test does not appear to be useful in predicting shoulder periprosthetic joint infections, Int. Orthop., 44, 1023–1029, https://doi.org/10.1007/s00264-020-04532-x, 2020.
Whiting, P. F., Rutjes, A. W. S., Westwood, M. E., Mallett, S., Deeks, J. J., Reitsma, J. B., Leeflang, M. M. G., Sterne, J. A. C., Bossuyt, P. M. M., and QUADAS-2 Group: QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies, Ann. Intern. Med., 155, 529–536, https://doi.org/10.7326/0003-4819-155-8-201110180-00009, 2011.
Wyatt, M. C., Beswick, A. D., Kunutsor, S. K., Wilson, M. J., Whitehouse, M. R., and Blom, A. W.: The Alpha-Defensin Immunoassay and Leukocyte Esterase Colorimetric Strip Test for the Diagnosis of Periprosthetic Infection: A Systematic Review and Meta-Analysis, J. Bone Joint Surg. Am., 98, 992–1000, https://doi.org/10.2106/JBJS.15.01142, 2016.
Xie, K., Qu, X., and Yan, M.: Procalcitonin and α-Defensin for Diagnosis of Periprosthetic Joint Infections, J. Arthroplasty, 32, 1387–1394, https://doi.org/10.1016/j.arth.2016.10.001, 2017.
Yu, B.-Z., Li, R., Fu, J., Chai, W., Hao, L.-B., and Chen, J.-Y.: Leukocyte esterase test and alpha-defensin test have similar accuracy for the diagnosis of periprosthetic joint infection, Int. Orthop., 45, 1677–1682, https://doi.org/10.1007/s00264-020-04903-4, 2021.
Yuan, J., Yan, Y., Zhang, J., Wang, B., and Feng, J.: Diagnostic accuracy of alpha-defensin in periprosthetic joint infection: a systematic review and meta-analysis, Int. Orthop., 41, 2447–2455, https://doi.org/10.1007/s00264-017-3647-3, 2017.
- Abstract
- Introduction
- Methods
- Results
- Discussion
- Limitations
- Conclusions
- Appendix A: Search strategies used for systematic review across PubMed, Embase, and Cochrane Library
- Code availability
- Data availability
- Author contributions
- Competing interests
- Ethical statement
- Disclaimer
- Acknowledgements
- Review statement
- References
- Abstract
- Introduction
- Methods
- Results
- Discussion
- Limitations
- Conclusions
- Appendix A: Search strategies used for systematic review across PubMed, Embase, and Cochrane Library
- Code availability
- Data availability
- Author contributions
- Competing interests
- Ethical statement
- Disclaimer
- Acknowledgements
- Review statement
- References