the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
In vitro mixing formulas for antimicrobial calcium sulfate beads for point-of-care applications: an additional update to formulas
Edward J. McPherson
Andrew J. Wassef
Matthew V. Dipane
Madhav Chowdhry
This communication updates a previous report describing mixing formulas for antimicrobial agents into a synthetic, dissolvable calcium sulfate (CaSO4) product to create small-sized beads (3–6 mm). Antimicrobial-loaded calcium sulfate (ALCS) beads are applied as surgeon-directed, point-of-care use in cases of periprosthetic joint infection (PJI), fracture-related infection (FRI), and other related bone site infections. This treatment technique has been utilized more frequently in the last decade to combat microbial musculoskeletal infections (MSIs). With the widening spectrum of identified microbes encountered in MSIs, we have provided additional antimicrobial formulas to be used singularly and in combination using our in vitro mixing lab protocol previously published. This communication reports 20 additional agents/combinations tested using our protocol to arrive at a standard mix formula or, when necessary, a modified mixing method. Most mixing formulas derived were for antimicrobials effective against fungi and mycobacterium species.
- Article
(2059 KB) - Full-text XML
- BibTeX
- EndNote
Musculoskeletal infections (MSIs) are increasingly challenging to treat. The reasons for this include the following: the many ongoing adaptive resistance mechanisms selected out by the persistent use of systemic, broad-spectrum antimicrobial agents, the increasing use of immune modulating agents deployed in the treatment of immune-related disorders, and the insertion of sophisticated metallic/ceramic implants for musculoskeletal reconstruction that can harbor micro-organisms (Kurtz et al., 2008; Hamad et al., 2022; Gallo et al., 2014). Treatment has required innovation. In the specific areas of periprosthetic joint infection (PJI), fracture-related infection (FRI), and other bone infection sites, one tactic used for treatment involves local delivery of antimicrobials via dissolvable antimicrobial-loaded calcium sulfate (CaSO4) in a small bead form (3–6 mm). The beads are made by the surgeon at the time of surgery, with point-of-care delivery to the infected site (McPherson et al., 2013; Abosala and Ali, 2020; Howlin et al., 2015).
In a previous article, we described mixing various antimicrobial agents into a synthetic, commercially pure CaSO4 product in a laboratory setting (McPherson et al., 2021; McPherson et al., 2022). In cursory review, this concept seems simple, but in reality, the process is tedious. The reported mixing formulas were developed to provide surgeons with access to the many available options for treatment. Because of a widening spectrum of identified microbes encountered in MSIs, we study additional agents singularly and in combination using our in vitro mixing lab protocol which simulates the operating room environment (Chowdhry et al., 2024; Nafea et al., 2024). This report provides 20 additional mixing formulas. We report antimicrobial doses used in our standardized mixing technique. Further, when encountering difficulty in mixing a specific agent(s), we developed a modified mixing technique to create the loaded bead.
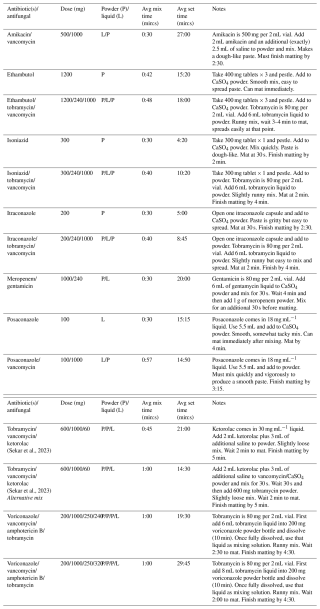
The study technique employed has been previously described in detail (McPherson et al., 2021). For each agent or combination evaluated, we performed the test a minimum of 3 times. The mix time and set time (defined below) for each agent/combination was averaged for the reported result. The CaSO4 product used for study was Synthecure® (Austin Medical Ventures, Memphis, TN), a pharmaceutically derived product. We used the 10 cm3 bead kit that consisted of CaSO4 hemihydrate powder that is mixed with 5 cm3 of sterile saline. When mixed, the unloaded CaSO4 product makes a paste volume of 10 cm3. The mixed product is spread into a sterile silicone bead mat, allowing for the fabrication of 3, 4.8, or 6 mm beads. For our study, we selected the 4.8 mm bead size. The bead mat and bead product formed are shown in Fig. 1.
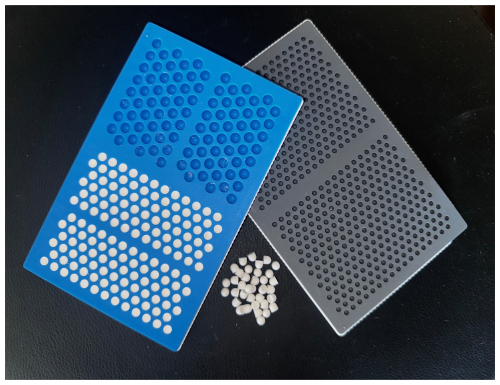
Figure 1Photograph of CaSO4 antimicrobial bead mat. The antimicrobial-loaded CaSO4 paste is placed into the bead mat, creating either 3.0, 4.8, or 6.0 mm beads. For study, the paste was spread into the 4.8 mm hemisphere molds. The bead mat was left on the countertop to set, as it would in the operating room. Time measurements were recorded to establish a set time for each formulation.
In the majority of antimicrobial formulae, the sterile powdered form of the agent was pre-mixed into the CaSO4 powder, after which the sterile saline was added. Mixing was performed in the 6.5 cm diameter mixing bowl with a stir stick (components provided in the kit) until a paste consistency devoid of clumps was achieved. The paste product was transferred via the spatula to the bead mat. We measured the time from initial mix until the product became consistently smooth for matting (defined as mix time). Once spread into the bead mat, the product was left to set on the laboratory table in standard conditions of 21.1 °C (70 °Fahrenheit) and 40 % humidity. We defined the product as “set” when we could bend the bead mat as it was turned upside down and the beads would fall onto the table. A second confirmatory test included a digital bead compression test when the bead was on the table. When “fully set”, the bead would not collapse by compressing the bead with the tip of the index finger. The measured “set time” was defined as the time from initial mix until the bead dropped out of the bead mat and was confirmed as fully set via the digital compression test.
Exceptions to the powder mix method noted above were liquid tobramycin and gentamicin. In many centers, both tobramycin and gentamicin come conveniently pre-mixed in 2 mL vials and can be used as a substitute to the sterile saline that is included in the kit. Liquid tobramycin (or gentamicin) provides an advantage in that other antimicrobial powders can be added to the CaSO4 powder, allowing combination formulas to be created.
During the standardized mix tests, we found difficulty in mixing numerous antimicrobial agents. In these cases, we encountered long set times, ranging from 30 min to over 3 h. We also experienced antimicrobial/CaSO4 combinations that set too quickly to fabricate a quality bead product. For these irregular set times, we experimented with alternative mixing methods with minimal dose alterations. We arrived at modified mixing techniques to provide reasonable set times, for all of these antimicrobial agents. The modified mixing instructions are described for each specific mix, with individualized mixing notes. The modified mixing instructions are grouped into the category of “modified-mix protocols”.
The standard mix times creating antimicrobial-loaded calcium sulfate (ALCS) beads are reported in Table 1. The two measured times include the mix time and set time. In the cases where liquid agents were substituted for saline, we also report mix time and set time in a similar fashion. For the standard mix method, all tested antimicrobial(s) were set within 4 to 13 min. The recorded mix times and set times tested between testing authors varied no more than 60 s in all agents tested.
Table 1Synthecure set times with antimicrobial/combinations – standard mix technique.
n/a – not applicable.
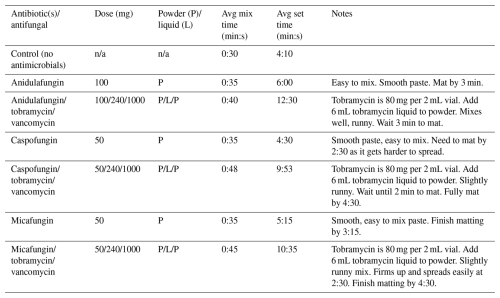
The modified mix protocols and described methods are reported in Table 2. For each agent/combination, we describe the modified mixing technique. The mix times and set times using the modified mix protocols are recorded. The recorded mix times and set times between the two testing authors varied no more than 60 s in all agents tested.
This supplemental compendium of mixing formulas is by no means a recommended treatment algorithm for MSIs. However, use of local antimicrobial delivery via ALCS beads is described globally (Tang et al., 2022; Kallala et al., 2018; Sahoo et al., 2024; Ferrando et al., 2017; Whitlark et al., 2016; Healy et al., 2012). Further, there are recent concerns by musculoskeletal infection experts that overuse of systemic antimicrobial agents adversely affects the human microbiome, creating concerning downstream consequences to the human host (Patangia et al., 2022; Aboushaala et al., 2023; Ribeiro et al., 2020). Local antimicrobial delivery may become a focus area for future MSI treatment. Studies are slowly emerging that show local delivery via ALCS beads may reduce the duration of parenteral antimicrobial therapy (McPherson et al., 2013). Until recently, antibiotic and antifungal formulas used to make ALCS beads have been within the purview of surgeon-to-surgeon or surgeon-to-company representative sidebar discussions. We bring these discussions to the forefront and include these additional formulas for ongoing academic debate. Moreover, we want to educate physicians treating bone and joint infections that these antimicrobial formulas exist. Physicians uninformed with this technique of antimicrobial delivery may potentially over-treat with adjuvant parenteral antimicrobials, causing untoward patient side effects. The description of these mixing formulas serves as a starting basis for discussion, providing a reference point for set times.
Since we first reported upon mixing formulas for antimicrobial-loaded CaSO4 beads, multiple CaSO4 bead products have emerged for clinical application. We highlight that every CaSO4 product sold for medical use as a bead product is unique and that the mixing formulas we have reported previously and here within are specific to the Synthecure product. Although generally applicable, our mixing data cannot be uniformly translated to other products. For mined and refined products, impurities (including heavy metal elements) impart variations in set times and also limit the mixing of many antimicrobials/combinations (Mu et al., 2019; Fernandes et al., 2021). For synthetic products, the proprietary formulas created provide efficient CaSO4 setting with antimicrobial agents and are unique to each product. The chemical reactions affecting these differences are not exactly known, but, empirically, product development has focused on efficient antimicrobial loading and elution. Each product is proprietary with unique performance (McPherson et al., 2022). We acknowledge that there are various pharmaceutical suppliers of antimicrobial agents, and mixing set times may vary slightly based upon added excipients. This study does not report upon elution of these agents from mixed beads and will require further testing. In a simulated in vitro synovial large joint model, we have documented high effective antimicrobial levels of vancomycin and tobramycin that are sustained for weeks (McPherson et al., 2022). However, for this study, we have not yet measured the effective antimicrobial levels of these reported agents in this model. This will be a topic of future study. We also warn that successful mixing and setting of antimicrobial agents is impaired when using hybrid bead products that contain CaSO4 in combinations with hydroxyapatite and/or β-tricalcium phosphate. In such products, the chemical interactions become complex, and from anecdotal experience, these additional agents distinctly impair mixing, setting, elution, and bead resorption. Finally, it is unlikely that novel carrier agents will enter the market in the near future due to the inherent challenges globally to approve a delivery agent. Thus, we advocate focused study and scrutiny of calcium sulfate for local antimicrobial delivery to musculoskeletal infection sites.
It remains our future goal to establish an open-access site such that all physicians and surgeons worldwide can report upon mixing formulas to educate and assist other physicians with difficult infection cases. Additionally, we hope to one day see the expansion of mixing formulas that extend beyond the current realm of antibiotic and antifungal chemicals and transition to non-specific antimicrobial agents.
We performed a mixing lab study describing 20 additional mixing formulas with mixing/set times in creating antimicrobial CaSO4 beads used for local delivery in periprosthetic joint infection, fracture-related infections, and other bone site infections. Most new formulas were focused on antifungal and antimycobacterial therapy. This information is to be utilized as an open-access reference for all physicians involved in the treatment of musculoskeletal infection.
All data in this study can be found in Tables 1 and 2.
EJM: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing original draft, supervision, project administration, writing (reviewing and editing). AJW: methodology, validation, investigation, resources, data curation, writing (reviewing and editing). MC: methodology, validation, investigation, software, formal analysis, data curation, writing original draft, writing (reviewing and editing). MVD: methodology, validation, formal analysis, investigation, resources, data curation, writing (reviewing and editing), project administration.
Edward J. McPherson has these stated conflicts: he is a speaker for Miller Orthopaedic Review Course (US and International) as part of Journal of Bone and Joint Surgery. He is a speaker and consultant for Zimmer-Biomet (Warsaw, IN, USA) and receives royalties for the ARCOS revision hip system. He is a speaker and consultant for Heraeus (Hanau, Germany) and receives royalties for Synthecure. He is a speaker for BoneSupport (Lund, Sweden).
Andrew J. Wassef has these stated conflicts: he is a speaker and consultant for Stryker (Mawah, NJ, USA). He is a speaker and consultant for Heraeus (Hanau, Germany) and receives royalties for Synthecure. He is a speaker for BoneSupport (Lund, Sweden).
No animals or humans were involved in the study. This was an in vitro (lab) study. Ethical clearance was waived for this study.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This paper was edited by Fintan Moriarty and reviewed by three anonymous referees.
Abosala, A. and Ali, M.: The Use of Calcium Sulphate beads in Periprosthetic Joint Infection, a systematic review, J. Bone Joint Infect., 5, 43–49, https://doi.org/10.7150/jbji.41743, 2020.
Aboushaala, K., Wong, A. Y. L., Barajas, J. N., Lim, P., Al-Harthi, L., Chee, A., Forsyth, C. B., Oh, C.-d., Toro, S. J., Williams, F. M. K., An, H. S., and Samartzis, D.: The Human Microbiome and Its Role in Musculoskeletal Disorders, Genes, 14, 1937, https://doi.org/10.3390/genes14101937, 2023.
Chowdhry, M., Dipane, M. V., Duncan, S. T., Pena, D., Stavrakis, A., and McPherson, E. J.: Next generation sequencing identifies an increased diversity of microbes in post lavage specimens in infected TKA using a biofilm disrupting irrigant, Knee, 51, 231–239, https://doi.org/10.1016/j.knee.2024.09.010, 2024.
Fernandes, G., Abhyankar, V., and O'Dell, J.: Calcium Sulfate as a Scaffold for Bone Tissue Engineering: A Descriptive Review, J. Dent., 9, 1–22, https://doi.org/10.15226/jdodt.2021.001124, 2021.
Ferrando, A., Part, J., and Baeza, J.: Treatment of Cavitary Bone Defects in Chronic Osteomyelitis: Bioactive glass S53P4 vs. Calcium Sulphate Antibiotic Beads, J. Bone Joint Infect., 2, 194–201, https://doi.org/10.7150/jbji.20404, 2017.
Gallo, J., Holinka, M., and Moucha, C. S.: Antibacterial surface treatment for orthopaedic implants, Int. J. Mol. Sci., 15, 13849–13880, https://doi.org/10.3390/ijms150813849, 2014.
Hamad, C., Chowdhry, M., Sindeldecker, D., Bernthal, N. M., Stoodley, P., and McPherson, E. J.: Adaptive antimicrobial resistance, a description of microbial variants, and their relevance to periprosthetic joint infection, Bone Joint J, 104-B, 575–580, https://doi.org/10.1302/0301-620X.104B5.BJJ-2021-1759.R1, 2022.
Healy, A. H., Reid, B. B., Allred, B. D., and Doty, J. R.: Antibiotic-Impregnated Beads for the Treatment of Aortic Graft Infection, Ann. Thorac. Surg., 93, 984–985, https://doi.org/10.1016/j.athoracsur.2011.07.066, 2012.
Howlin, R. P., Brayford, M. J., Webb, J. S., Cooper, J. J., Aiken, S. S., and Stoodley, P.: Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections, Antimicrob Agents Chemother, 59, 111–120, https://doi.org/10.1128/AAC.03676-14, 2015.
Kallala, R., Harris, W. E., Ibrahim, M., Dipane, M., and McPherson, E.: Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty, Bone Joint Res., 7, 570–579, https://doi.org/10.1302/2046-3758.710.Bjr-2017-0319.R1, 2018.
Kurtz, S. M., Lau, E., Schmier, J., Ong, K. L., Zhao, K., and Parvizi, J.: Infection burden for hip and knee arthroplasty in the United States, J. Arthroplasty, 23, 984–991, https://doi.org/10.1016/j.arth.2007.10.017, 2008.
McPherson, E., Dipane, B. A., and Sherif, M. D.: Dissolvable Antibiotic Beads in Treatment of Periprosthetic Joint Infection and Revision Arthroplasty – The Use of Synthetic Pure Calcium Sulfate (Stimulan®) Impregnated with Vancomycin & Tobramycin, Reconstructive Review, 3, 27, https://doi.org/10.15438/rr.v3i1.27, 2013.
McPherson, E. J., Dipane, M. V., Chowdhry, M., and Wassef, A. J.: Fabrication of antibiotic-loaded dissolvable calcium sulfate beads: an in vitro mixing lab utilizing various antibiotic mixing formulas, J. Bone Joint Infect., 6, 405–412, https://doi.org/10.5194/jbji-6-405-2021, 2021.
McPherson, E. J., Jennings, J. A., Yunis, O., Harris, M. A., Dipane, M. V., Curtin, N. L., Chowdhry, M., Wassef, A. J., Bumgardner, J. D., and Noel, S. P.: Simulated large joint fluid model for evaluating intra-articular antibiotic delivery systems: initial evaluation using antibiotic-loaded calcium sulfate beads, J. Bone Joint Infect., 7, 117–125, https://doi.org/10.5194/jbji-7-117-2022, 2022.
Mu, X., Zhu, G., Li, X., Li, S., Gong, X., Li, H., and Sun, G.: Effects of Impurities on CaSO4 Crystallization in the Ca(H2PO4)2–H2SO4–H3PO4–H2O System, ACS Omega, 4, 12702–12710, https://doi.org/10.1021/acsomega.9b01114, 2019.
Nafea, A. M., Wang, Y., Wang, D., Salama, A. M., Aziz, M. A., Xu, S., and Tong, Y.: Application of next-generation sequencing to identify different pathogens, Front. Microbiol., 14, 1329330, https://doi.org/10.3389/fmicb.2023.1329330, 2024.
Patangia, D. V., Anthony Ryan, C., Dempsey, E., Paul Ross, R., and Stanton, C.: Impact of antibiotics on the human microbiome and consequences for host health, Microbiologyopen, 11, e1260, https://doi.org/10.1002/mbo3.1260, 2022.
Ribeiro, C. F. A., Silveira, G. G. d. O. S., Cândido, E. d. S., Cardoso, M. H., Espínola Carvalho, C. M., and Franco, O. L.: Effects of Antibiotic Treatment on Gut Microbiota and How to Overcome Its Negative Impacts on Human Health, ACS Infect. Dis., 6, 2544–2559, https://doi.org/10.1021/acsinfecdis.0c00036, 2020.
Sahoo, S. S., Biswas, M., Kumar, A., Sahoo, B., Mandal, S., and Saha, S.: Unusual Sites of Brodie's Abscess and the use of Calcium Sulfate Beads: A Case Series, J. Orthop. Case Rep., 14, 110–115, https://doi.org/10.13107/jocr.2024.v14.i11.4932, 2024.
Sekar, A., Gil, D., Tierney, P. A., McCanne, M., Daesety, V., Trendafilova, D., Muratoglu, O. K., and Oral, E.: Synergistic use of anti-inflammatory ketorolac and gentamicin to target staphylococcal biofilms, Research Square [preprint], https://doi.org/10.21203/rs.3.rs-3471646/v1, 2023.
Tang, X., Li, J., Wang, C., Liu, F., Guo, J., Tan, J., Song, Q., Cao, H., and Zhang, Y.: Antibiotic-loaded calcium sulfate beads in spinal surgery for patients with spondylodiscitis: a clinical retrospective study, BMC Musculoskel. Dis., 23, 270, https://doi.org/10.1186/s12891-022-05230-y, 2022.
Whitlark, J. D., Kirollos, J. A., and Jackson, S. M.: Simplified Method for Treating Osteomyelitis of the Sternoclavicular Joint, Ann. Thorac. Surg., 101, 1211–1212, https://doi.org/10.1016/j.athoracsur.2015.08.053, 2016.