the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Differential synovial fluid white blood cell count for the diagnosis of chronic peri-prosthetic joint infection – a systematic review and meta-analysis
Martin Clauss
Ana Ribau
Ricardo Sousa
Introduction: Peri-prosthetic joint infection (PJI) is a significant complication of arthroplasty, lacking a single gold standard diagnostic test. Synovial fluid white blood cell (WBC) count and polymorphonuclear neutrophil (PMN) proportion are widely used diagnostic tools, but their optimal cutoffs remain unclear, particularly for chronic PJI. Material and methods: This systematic review and meta-analysis included 74 studies published between 2000 and 2024. Data on diagnostic performance (sensitivity, specificity, and diagnostic odds ratios – DORs) of WBC count and PMN proportions were analysed. Sub-group analyses and heterogeneity assessments were performed, and optimal cutoffs for diagnostic accuracy were identified. Results: The meta-analysis revealed a WBC count summary DOR of 58.38 (95 % CI – confidence interval: 48.48–70.32) with an area under the curve (AUC) of the summarized receiver operating characteristic curve of 0.952. The PMN proportion showed a DOR of 43.17 (95 % CI: 35.31–52.79) and an AUC of 0.941. Optimal diagnostic thresholds for chronic PJI were WBC count > 2600 cells per microlitre and PMN > 70 %. Rule-in thresholds (specificity >95 %) were WBC count ≥ 3000 cells per microlitre and PMN ≥ 75 %, while rule-out thresholds (sensitivity > 95 %) were WBC count ≤ 1500 cells per microlitre and PMN ≤ 65 %. Confounding conditions such as fractures, inflammatory arthritis, and metal-related reactions reduced test accuracy. Conclusions: Synovial fluid analysis remains a critical diagnostic tool for chronic PJI. Thresholds of WBC count < 1500 and >3000 cells per microlitre and PMN < 65 % and >75 % provide reliable negative and positive predictive values. A standardized diagnostic framework is essential for addressing remaining controversies and ensuring consistent interpretation across clinical settings.
- Article
(2044 KB) - Full-text XML
- Comment
-
Supplement
(578 KB) - BibTeX
- EndNote
Peri-prosthetic joint infection (PJI) is a potentially dismal complication of total joint arthroplasty. As the expected number of arthroplasties is rising worldwide, so, too, is the burden of the small but consistent infection rate (Premkumar et al., 2021; Rupp et al., 2020). In fact, infection is a leading cause of total joint replacement failure, and it is critical to accurately diagnose it, as failure to do so will hamper treatment success (Sousa et al., 2023). Still, there is no available gold standard diagnostic test on its own. The definition of PJI thus relies on the combined interpretation of multiple different tests (Osmon et al., 2013; Parvizi and Gehrke, 2014; Shohat et al., 2019; Sousa et al., 2023; Workgroup Convened by the Musculoskeletal Infection Society, 2011).
Synovial fluid differential white blood cell (WBC) count is a widely available and rather inexpensive test that has long been recognized as a critical part of the preoperative workup before revision arthroplasty. Its accuracy for the diagnosis of PJI in the setting of chronically painful joints has been studied extensively and gives consistently good results.
There are, nevertheless, open questions. First, different proposed cutoffs exist due to variations in PJI definitions and joint-specific studies. Second, conditions such as peri-prosthetic fractures, inflammatory arthritis, crystal arthropathy, or metal-ion-related tissue reactions can elevate WBC counts irrespective of infection, affecting accuracy. Third, the role of differential WBC count in acute postoperative PJI remains less clear due to post-surgical inflammation.
This systematic review aimed to (1) determine the overall diagnostic accuracy of synovial WBC counts and polymorphonuclear neutrophil (PMN) proportions for chronic PJI, (2) establish optimal interpretation cutoffs, and (3) evaluate test performances under confounding conditions.
This systematic review adheres to PRISMA of Diagnostic Test Accuracy Studies (PRISMA-DTA) (McInnes et al., 2018).
2.1 Search strategy
A computer-aided search on MEDLINE and EMBASE (1 January 2000–1 February 2024) identified studies evaluating synovial WBC count and PMN proportion for PJI diagnosis. Reference lists of included articles were also reviewed. The search strategy combined terms related to arthroplasty infection, synovial fluid diagnosis, and diagnostic accuracy (see Table S1 in the Supplement).
2.2 Inclusion and exclusion criteria
Studies were included if they evaluated synovial fluid WBC count and/or PMN proportion in suspected PJI cases, provided diagnostic performance data, were conducted on humans ≥18 years old, and were published in English between 2000 and 2024. Only studies reporting results on chronic PJI were included. We excluded studies on infections unrelated to PJI, case reports, animal studies, and reviews.
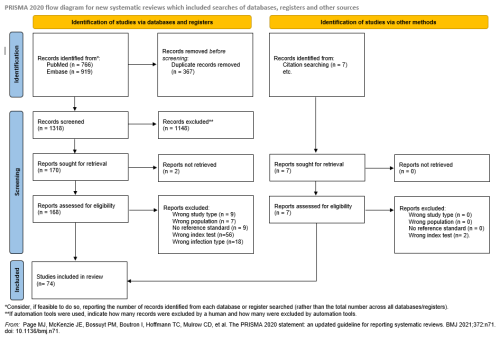
Duplicates were removed using Mendeley. Screening was performed with Rayyan software by two independent reviewers per article, first by title or abstract and then by full text. Disagreements were resolved by consensus. The study selection process is shown in Fig. 1 (Page et al., 2021).
2.3 Data extraction
Data were extracted in duplicate, resolving discrepancies by consensus. Collected data included study characteristics, diagnostic criteria, infection type, patient characteristics (e.g. inflammatory diseases, fractures, metallosis, or inflammatory arthritis), WBC count and PMN cutoffs, sensitivity, specificity, predictive values, area under the curve (AUC), accuracy, and Youden index. True positive, true negative, false positive, and false negative values were calculated.
2.4 Risk of bias and quality assessment
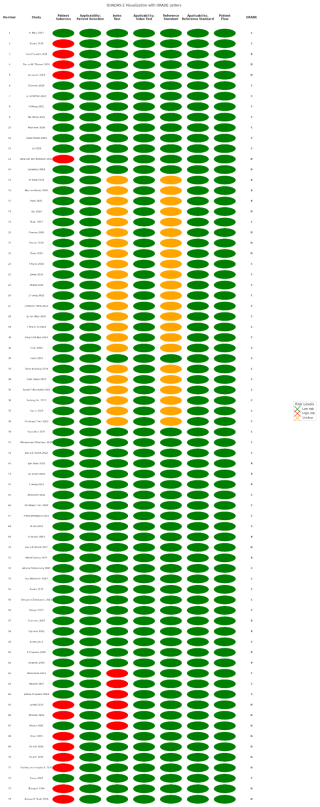
Two independent investigators per article assessed the risk of bias and applicability of each included study by using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) (Whiting et al., 2011) tool, with ratings of “low”, “high”, or “unclear”. Discrepancies were resolved by consensus.
Quality was assessed by two independent investigators per article with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool (Schünemann et al., 2023). This tool is used to evaluate the quality of the evidence and the strength of the recommendations. Studies were classified as “high (A)”, “moderate (B)”, “low (C)”, or “very low (D)” quality.
2.5 Information synthesis and statistical analysis
With the information extracted, we calculated the diagnostic odds ratio (DOR) for every study. Although sensitivity and specificity are commonly used in diagnostic test research due to their clinical relevance, these metrics can introduce a threshold effect when multiple studies are compared. Although the DOR has limited direct clinical application, it is particularly useful in systematic reviews of diagnostic accuracy because it reflects the overall effectiveness of a diagnostic test (Arias and Molina, 2015; De Sousa et al., 2009). We used DORs along with 95 % confidence intervals (CIs) and summarized receiver operating characteristic (sROC) curves as part of the meta-analysis (De Sousa et al., 2009). The analyses were conducted by using a random-effects model and the DerSimonian–Laird approach. In addition, a sub-group analysis was performed based on the joint involved (hip or knee).
Heterogeneity in the reported sensitivities and specificities was assessed by using Higgins' I2 statistic. All statistical analyses were performed with Jamovi (version 2.2.5) and Meta-disc (versions 1–4).
2.6 Cutoff determination
To determine interpretation cutoff values, we used only those articles that calculated their own ideal cutoff based on their data and excluded all papers that used pre-determined cutoffs. We excluded from this analysis studies that focused on patients with confounding circumstances such as fractures or dislocation, inflammatory arthritis, crystal arthropathy, or adverse local tissue reactions due to metal ions.
We used three different data interpretation strategies: (1) the “optimal” cutoff based on the best combination of specificity and sensitivity, (2) a “rule-in” cutoff that focused on optimizing specificity, and (3) a “rule-out” cutoff that focused on optimizing sensitivity.
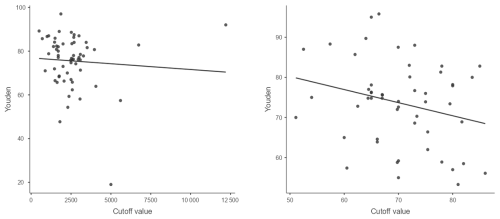
We used the Youden index and extracted cutoffs and presented them in a scatterplot diagram. The optimal cutoff was determined by using median values. We obtained optimal cutoffs for combined total knee arthroplasty (TKA) and total hip arthroplasty (THA) as well as for TKA and THA individually.
To estimate an ideal rule-in/rule-out threshold, we decided to use only specificity or sensitivity values of >95 %. We used specificity or sensitivity vs. extracted cutoffs and presented them in a scatterplot diagram, and the rule-in/rule-out cutoff was determined using median values. We obtained the rule-in/rule-out value for the combined TKA and THA. There were not enough data to extract a rule-in value for TKA and THA individually.
We performed a post hoc sub-analysis by sub-dividing groups between the modern or more sensitive PJI definition criteria (International Consensus Meeting ICM, European Bone and Joint Infection Society EBJIS, and Infectious Disease Society of America IDSA) and the old PJI definition criteria (Musculoskeletal Infection Society MSIS and clinical outcome) to analyse the sensitivity and specificity cutoff values of these two groups. Information concerning other joints (e.g. shoulders, elbows, hemi-hips, uni-knees, or ankles) was too scarce to merit meaningful calculations.
3.1 Search results
The search identified 1685 records. After removing duplicates, 1318 studies underwent title and abstract screening. Of 170 full-text studies assessed, 96 were excluded for reasons such as wrong study type (9), incorrect population (7), unspecified reference standard (9), different index test (56), or lack of chronic PJI separation (18). Five additional studies were identified through bibliography review, yielding 74 included studies (Fig. 1).
3.2 Risk of bias and quality assessment
Results from QUADAS-2 (Whiting et al., 2011) are presented in Fig. 2. Most studies had a high risk of bias but good applicability, largely due to retrospective study designs. Patient selection was unclear in more than 10 studies, while index test and reference standard issues were found in more than 20 studies.
GRADE assessment classified 13 studies (17.6 %) as B, 43 (58.1 %) as C, and 18 (24.3 %) as D (Fig. 2). Because of the retrospective nature of the majority of the papers, more than 50 % of the studies included were classified as GRADE C.
3.3 Characteristics of the studies
We included 74 studies (Table 1). The publication years ranged from 2003 to 2024, the year with the highest publication rate being 2023 with 13 papers. Twenty-six papers were prospective cohorts (35.1 %) and 48 were retrospective cohorts (64.9 %), covering 18 960 patients (19 to 4462). Papers used different PJI reference standards: 14 used clinical definitions (18.9 %), 3 the IDSA 2013 definition (4.1 %), 35 the MSIS 2013 definition (47.3 %), 17 the ICM 2018 definition (23 %), and 4 the EBJIS 2021 definition (5.4 %). One paper combined MSIS 2013 and ICM 2018.
We extracted 135 different cutoffs for synovial WBC count and 114 different cutoffs for synovial PMN. The distribution by joint for synovial WBC count was 63 for hips and knees together, 28 for knees only, 31 for hips only, 4 for shoulders only, and 7 for all four joints together. The distributions by joint for synovial polymorphonuclear proportion (PMN %) were 52, 29, 29, 2, and 4, respectively. There was also one study that focused on unicompartmental knee replacements (Schwartz et al., 2012), one on hip hemiarthroplasties (Salimy et al., 2024), and one on total ankle replacement (Thiesen et al., 2019), which provided information on synovial WBC count and PMN % (Table 2).
Table 2Proposed interpretation cutoffs for joints other than TKA and THA.
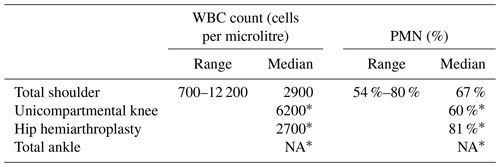
* Only one paper for this condition. TKA: total knee arthroplasty. THA: total hip arthroplasty. WBC: white blood cell. PMN %: polymorphonuclear proportion. NA: not available.
Considering all papers with no sub-groupings at all, the proposed optimal cutoffs varied widely between 700 and 19 240 cells per microlitre and between 53 % and 99 % PMN. Median values were 3000 cells per microlitre and 75.4 % PMN for all joints and all conditions. Median values for knees, hips, and shoulders separately were 3000 cells per microlitre and 71.5 % PMN, 3000 cells per microlitre and 80 % PMN, and 2900 cells per microlitre and 67 % PMN, respectively.
3.4 Meta-analysis
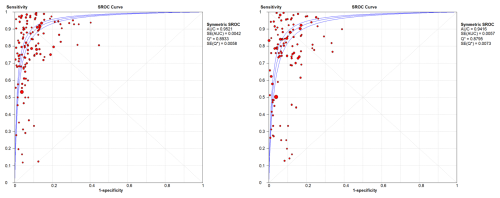
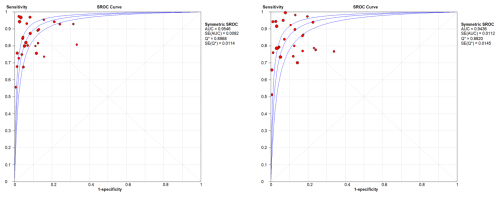
WBC count showed a summary DOR of 58.38 (95 % CI: 48.48–70.32), an AUC of the sROC of 0.952 (SE: 0.0042), and a Q index of 0.893. PMN proportion showed a summary DOR of 43.17 (95 % CI: 35.31–52.79), an AUC of the sROC of 0.941 (SE: 0.057), and a Q index of 0.879 (Fig. 3).
The heterogeneities (I2) of sensitivity and specificity for WBC count were 99.3 % and 88.3 %, respectively, and for PMN % they were 93.9 % and 88.5 %, respectively. DOR heterogeneity was 77.4 % for WBC count and 77.9 % for PMN %.
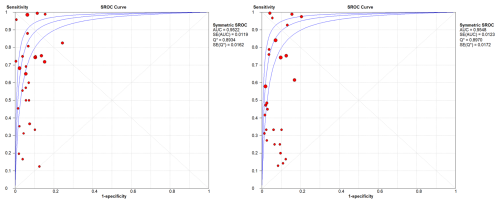
In the sub-group analysis by joint, knees presented a WBC count summary DOR of 83.1 (95 % CI: 54.72–126.21), an AUC of the sROC of 0.954 (SE: 0.0082), a Q index of 0.896, a PMN proportion summary DOR of 63.29 (95 % CI: 38.62–103.74), an AUC of the sROC of 0.936 (SE: 0.011), and a Q index of 0.882. In hips, results showed a WBC count summary DOR of 33.58 (95 % CI: 21.44–52.63), an AUC of the sROC of 0.952 (SE: 0.019), a Q index of 0.893, a PMN proportion summary DOR of 27.12 (95 % CI: 16.22–45.35), an AUC of the sROC of 0.954 (SE: 0.012), and a Q index of 0.897 (Figs. 4 and 5).
3.5 Cutoff determination
In the chronic stage, when no confounding circumstances were present, and excluding papers that used pre-determined cutoffs, the suggested cutoffs varied between 1100 and 5000 cells per microlitre and 53 % and 86 % PMN. The optimal cutoff values were 2537 cells per microlitre for knees, 2696 cells per microlitre for hips, and 70 % PMN for both (Fig. 6, Table 3).
Rule-in cutoffs of ≥2700 cells per microlitre and ≥75 % consistently demonstrated over 95 % specificity, and rule-out cutoffs of ≤1640 cells per microlitre and ≤66 % exceeded 95 % sensitivity for both hips and knees. There was not enough information to recommend a different cutoff for joints other than TKA and THA (Table 3).
When sub-dividing groups between more sensitive PJI definition criteria (ICM, EBJIS, and IDSA; Group 1) and other PJI definition criteria (MSIS and clinical outcome; Group 2), we could see that optimal cutoffs were very similar between them. However, when we applied a rule-in/rule-out calculation, Group 2 presented insufficient data in some cases due to the small number of papers with a specificity or sensitivity of >95 % (Table 4).
Table 4Cutoffs in the modern or more sensitive PJI definition (Group 1) and in the older PJI definition (Group 2).
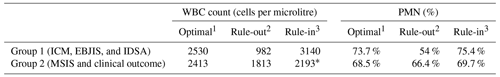
1 Defined by the best combination of sensitivity and specificity. 2 Defined by a sensitivity of >95 %. 3 Defined by a specificity of >95 %. * No papers with >95 % specificity in THA, leaving a reduced sample to compare with other calculations.
3.6 Confounding conditions
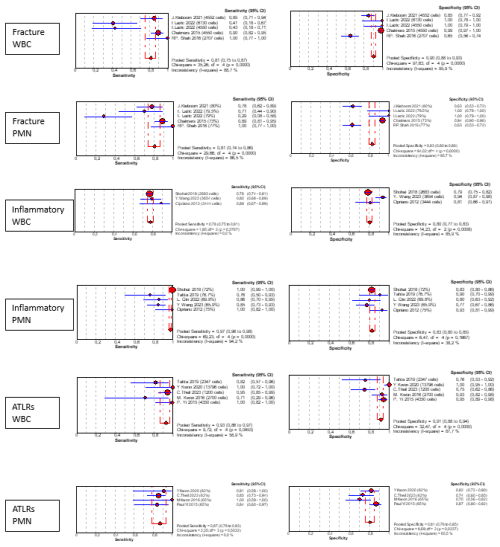
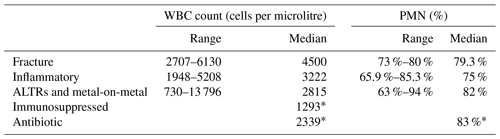
Some papers were focused on specific conditions that can modify synovial cellularity and PMN %, the so-called confounding conditions. We found six different conditions: five papers were specific to peri-prosthetic fracture associated with PJI (Chalmers et al., 2014; van den Kieboom et al., 2021; Lazic et al., 2022a, b; Shah et al., 2016), six papers to inflammatory conditions (Cipriano et al., 2012; Qin et al., 2022; Ren et al., 2022; Shohat et al., 2018; Tahta et al., 2019; Wang et al., 2023), four papers to adverse local tissue reactions due to head–neck taper corrosion or metal-on-metal bearings (Kwon et al., 2016, 2020; Theil et al., 2024; Yi et al., 2015), one paper to immunosuppressed patients (Lazarides et al., 2019), and one paper to antibiotic use (Salimy et al., 2023).
The cutoff variability and medians of confounding conditions are presented in Table 5. We were not able to replicate our cutoff interpretation strategy due to the small sample size, but Fig. 7 shows a rough estimate of the diagnostic accuracy in each scenario.
Accurate PJI diagnosis is challenging, as there is no perfect gold standard test. Definitive diagnosis must therefore rely on a set of pre-determined criteria that include both preoperative and intraoperative findings such as microbiological and histological results. In the past decade, several different PJI definitions have been proposed, which have all included synovial fluid examination (Mcnally et al., 2021; Osmon et al., 2013; Shohat et al., 2019; Workgroup Convened by the Musculoskeletal Infection Society, 2011). Although there is no universally accepted algorithm for diagnosis, it is indisputable that joint aspiration with a differential synovial fluid WBC count is a critical part of current diagnosis.
Our systematic review confirms its value. The DOR and sROC results obtained in our meta-analysis demonstrated that both tests have a high diagnostic accuracy and are powerful tools for reaching PJI diagnosis. On the basis of our results, WBC count analysis demonstrated the highest DOR and sROC values compared with those for PMN proportion. In addition to the high diagnostic accuracy of synovial fluid analysis, it is both inexpensive and widely available. We believe it should therefore continue to be a critical part of any diagnostic flowchart and definition criteria.
Still, there are a number of open questions that we were not able to address entirely in this paper. First, the role of synovial WBC and PMN proportion analysis in the immediate postoperative period is not as well studied. After joint replacement surgery, there is a rise in both WBC count and PMN proportion that takes several weeks to normalize despite an uneventful course (Christensen et al., 2013). We found a number of studies that focused on acute postoperative PJI, but they lacked consistency in how to define infection or even in what timing constitutes the acute period, and therefore we chose to analyse them separately in a different paper.
A different controversy that we were not able to clarify completely is whether the affected joint has an effect on the optimal threshold of the WBC count and/or PMN proportion. The vast majority of papers we found focused on TKA and/or THA separately or combined. We did not find significant differences to recommend adopting different thresholds for hips and knees. In addition, there is not enough available evidence to recommend alternative cutoffs for joints other than TKA and THA.
Though it is not controversial that total WBC count and PMN proportion are highly valuable tests in the diagnostic workup for chronic PJI, still lacking is a universal consensus on what the ideal interpretation thresholds are. Many of the papers that examine the performance of these tests simply adopt previously proposed cutoffs and report on their accuracy in a specific cohort. Many others analyse their own population, adopt certain diagnostic criteria to serve as the PJI gold standard, and offer optimal cutoffs for interpretation. These results are influenced by variables such as population characteristics (e.g. PJI prevalence), a PJI definition standard, or even different laboratory (e.g. microbiology) standards that greatly influence final results. That being said, despite the wide range of proposed cutoffs, we were able to find some consistent results.
The optimal threshold, defined as the median of proposed cutoffs based on the best combination of specificity and sensitivity, was found to be around 2600 cells per microlitre and 70 % PMN overall. Nevertheless, we aimed to find a rule-in threshold by focusing on optimizing specificity at >95 % and found that it was slightly higher (both for WBC count and PMN %). We recommend the 3000 cells per microlitre (sensitivity 88.7 %, specificity 98.5 %) and 75 % PMN (sensitivity 77 %, specificity 97.8 %) thresholds to support the infection diagnosis and definition, as these figures are easy to recall and are compatible with both previously proposed cutoffs and results specifically in papers that use more sensitive or modern definitions (i.e. EBJIS 2021, ICM 2018, and IDSA). On the other hand, when one is looking to rule out PJI during the diagnostic workup, we recommend adopting a lower threshold that optimizes sensitivity at >95 %. Following similar principles enunciated previously, we recommend that infection be suspected and other diagnostic test(s) performed with a WBC count of over 1500 cells per microlitre (sensitivity 100 %, specificity 88 %) and 65 % PMN (sensitivity 95.8 %, specificity 78.6 %).
Lastly, it is crucial to acknowledge that there are special confounding circumstances that hamper the diagnostic accuracy of these tests. Previous antibiotic therapy is associated with a high number of false negative results (Salimy et al., 2023; Shahi et al., 2015; Trampuz et al., 2007). In the chronic setting, this limitation is simple to overcome by withholding antibiotics and repeating the joint tap at a later stage (at least 2 weeks after antibiotic discontinuation). Other pro-inflammatory conditions are perennial and may cause elevated WBC counts regardless of the presence of infection. These circumstances may be joint- or implant-related, e.g. associated peri-prosthetic fractures, dislocation, or metallosis, or may include systemic diseases such as inflammatory arthritis or crystal arthropathy. We found no evidence to justify adopting different condition-specific cutoffs, but information given by the WBC count and/or PMN proportion should be interpreted with caution, as diagnostic accuracy is inferior in these settings.
Synovial fluid WBC count is a crucial part of any PJI diagnostic workup and definition. In the absence of confounding circumstances, WBC counts of <1500 and >3000 cells per microlitre and proportions of PMN < 65 % and >75 % seem to have very good negative and positive predictive values, respectively. A uniform PJI diagnostic standard is required to facilitate studies able to address persistently open questions, such as the true optimal interpretation thresholds or the need for joint-specific or even condition-specific cutoffs.
Software code and data supporting the conclusions of this article will be made available by the authors on request.
The supplement related to this article is available online at https://doi.org/10.5194/jbji-10-165-2025-supplement.
Marjan Wouthuyzen-Bakker (Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands), Thomas Bauer (Department of Pathology, The Cleveland Clinic Foundation, Cleveland, OH, USA), Elie Berbari (Division of Public Health, Infectious Diseases and Occupational Medicine, Department of Medicine, Mayo Clinic College of Medicine and Science, Mayo Clinic, Rochester, MN, USA), Martin Clauss (Department of Orthopaedics and Trauma Surgery, University Hospital Basel, Basel, Switzerland), Nicolas Cortes-Penfield (Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, NE, USA), Matthew Dietz (Department of Orthopaedics, School of Medicine, West Virginia University, Morgantown, WV, USA), Jaime Esteban (Department of Medical Microbiology, IIS-Fundacion Jimenez Diaz, Madrid, Spain), Tristan Ferry (Infectious Diseases Department, Croix-Rousse Hospital, Hospices Civils de Lyon, Lyon, France), Thorsten Gehrke (Department of Orthopaedic Surgery, Endo-Klinik Hamburg, Specialist Clinic for Bone and Joint Surgery, Hamburg, Germany), Andor Glaudemans (Medical Imaging Center, Department of Nuclear Medicine & Molecular Imaging, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands), Benjamin Langworth (Division of Biostatistics and Health Data Science, School of Public Health, University of Minnesota, Rochester, MN, USA), Martin McNally (The Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford University Hospitals, Oxford, UK), Andy Miller (Department of Infectious Disease, Hospital for Special Surgery, New York, NY, USA), Sandra Nelson (Division of Infectious Diseases, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA), Javad Parvizi (Department of Orthopaedic Surgery, International Joint Center, Acibadem University Hospital, Istanbul, Türkiye), Robin Patel (Division of Infectious Diseases, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA), Holger Rohde (Institute of Medical Microbiology, Virology and Hygiene, University Medical Center Hamburg-Eppendorf, Hamburg, Germany), Thorsten Seyler (Department of Orthopaedics, Duke University Hospital, Durham, NC, USA), Irene Sigmund (Department of Orthopaedics and Trauma Surgery, Medical University of Vienna, Vienna, Austria), Alex Soriano (Department of Infectious Diseases, University of Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer, Hospital Clinic of Barcelona, CIBERINF Ciber in Infectious Diseases, Barcelona, Spain), and Ricardo Sousa (Department of Orthopedics, Centro Hospitalar Universitário de Santo António, Porto, Portugal) on behalf of the Leukocyte Count Synovial Fluid working group for the Unified PJI definition task force.
RS and MC designed the research. MSM, MC, AR, and RS conducted the screening and selection of the articles and the collection of the data. MSM performed the statistical analysis and wrote the first draft of the paper. MSM, MC, AS, and RS contributed to the writing of the paper. All the authors contributed to the data interpretation, revised each draft for important intellectual content, and read and approved the final manuscript.
Two of the (co-)authors are members of the editorial board of Journal of Bone and Joint Infection and are ExCom members (president Ricardo Sousa and vice-president Martin Clauss) of EBJIS. The other authors have no other competing interests to declare.
No ethical approval was needed for systematic review.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors would like to express their gratitude to Benjamin Langworthy at the Division of Biostatistics & Health Data Science at the University of North Carolina, Chapel Hill, for his advice on how to proceed with data interpretation.
This paper was edited by Anna Stefánsdóttir and reviewed by two anonymous referees.
Alkadhem, M. F., Ettema, H., Wagenmakers-Huizenga, L. M. F., Ploegmakers, J. J. W., Muller Kobold, A. C., Wouthuyzen-Bakker, M., and van Driel, P. B. A. A.: Synovial Calprotectin is Superior to Synovial Leukocyte Count in Excluding Chronic Periprosthetic Joint Infections, a Retrospective Cohort Study, J. Arthroplast., 39, 1926–1931, https://doi.org/10.1016/j.arth.2024.02.064, 2024.
Arias, M. M. and Molina, M.: El metaanálisis de pruebas diagnósticas, Pediatría Atención Primaria, 17, 281–285, https://doi.org/10.4321/S1139-76322015000400023, 2015.
Baker, C. M., Goh, G. S., Tarabichi, S., Shohat, N., and Parvizi, J.: Synovial C-Reactive Protein is a Useful Adjunct for Diagnosis of Periprosthetic Joint Infection, J. Arthroplas., 37, 2437–2443, https://doi.org/10.1016/j.arth.2022.06.016, 2022.
Balato, G., Franceschini, V., Ascione, T., Lamberti, A., Balboni, F., and Baldini, A.: Diagnostic accuracy of synovial fluid, blood markers, and microbiological testing in chronic knee prosthetic infections, Arch. Orthop. Trauma Surg., 138, 165–171, https://doi.org/10.1007/s00402-017-2832-6, 2018a.
Balato, G., Franceschini, V., Ascione, T., Lamberti, A., D'Amato, M., Ensini, A., and Baldini, A.: High performance of α-defensin lateral flow assay (Synovasure) in the diagnosis of chronic knee prosthetic infections, Knee Surgery, Sports Traumatology, Arthroscopy, 26, 1717–1722, https://doi.org/10.1007/s00167-017-4745-x, 2018b.
Berger, P., Van Cauter, M., Driesen, R., Neyt, J., Cornu, O., Bellemans, J., of Orthopaedic Surgery, P., of Orthopaedic, P., and Joint, B. J.: Diagnosis of prosthetic joint infection with alpha-defensin using a lateral flow device, Bone Joint J., 99-B, 1176–1182, https://doi.org/10.1302/0301-620X.99B9, 2017.
Bingham, J., Clarke, H., Spangehl, M., Schwartz, A., Beauchamp, C., and Goldberg, B.: The Alpha Defensin-1 Biomarker Assay can be Used to Evaluate the Potentially Infected Total Joint Arthroplasty, Clin. Orthop. Relat. Res., 472, 4006–4009, https://doi.org/10.1007/s11999-014-3900-7, 2014.
Bottagisio, M., Viganò, M., Pellegrini, A., Logoluso, N., Zagra, L., Prina, A., de Girolamo, L., and De Vecchi, E.: Evaluation of Synovial Calprotectin by Using a Lateral Flow Test for the Diagnosis of Prosthetic Joint Infections, Diagnostics, 13, 741, https://doi.org/10.3390/diagnostics13040741, 2023.
Burchette, D. T., Dasci, M. F., Fernandez Maza, B., Linke, P., Gehrke, T., and Citak, M.: Neutrophil–Lymphocyte Ratio and Lymphocyte–Monocyte Ratio correlate with Chronic Prosthetic Joint Infection but are not useful markers for diagnosis, Arch. Orthop. Trauma Surg., 144, 297–305, https://doi.org/10.1007/s00402-023-05052-0, 2024.
Cai, B. Y., Liang, J., Chen, X., Zhang, G., Jing, Z., Zhang, R., Lv, L., Zhang, W., and Dang, X.: Synovial fluid neutrophil extracellular traps could improve the diagnosis of periprosthetic joint infection, Bone Joint Res., 12, 113–120, https://doi.org/10.1302/2046-3758.122.BJR-2022-0391.R1, 2023.
Chalmers, P. N., Walton, D., Sporer, S. M., and Levine, B. R.: Evaluation of the role for synovial aspiration in the diagnosis of aseptic loosening after total knee arthroplasty, J. Bone Joint Surg., 97, 1597–1603, https://doi.org/10.2106/JBJS.N.01249, 2014.
Christensen, C. P., Bedair, H., Valle, C. J. D., Parvizi, J., Schurko, B., and Jacobs, C. A.: The natural progression of synovial fluidwhite blood-cell counts and the percentage of polymorphonuclear cells after primary total knee arthroplasty a multicenter study, J. Bone Joint Surg. Ser. A, 95, 2081–2087, https://doi.org/10.2106/JBJS.L.01646, 2013.
Cipriano, C. A., Brown, N. M., Michael, A. M., Moric, M., Sporer, S. M., and Della Valle, C. J.: Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis, J. Bone Joint Surg., 94, 594–600, https://doi.org/10.2106/JBJS.J.01318, 2012.
Cohen-Levy, W. B., Salimy, M. S., Lans, J., Canas, A. E., Melnic, C. M., and Bedair, H. S.: The Performance of Diagnostic Tests for Identifying Periprosthetic Joint Infection After Failed Partial Knee Arthroplasty, J. Arthroplast., 37, 2449–2454, https://doi.org/10.1016/j.arth.2022.06.021, 2022.
Denyer, S., Eikani, C., Sheth, M., Schmitt, D., and Brown, N.: Diagnosing periprosthetic joint infection a validation study of blood cell ratio combinations, Bone Joint Open, 4, 881–888, https://doi.org/10.1302/2633-1462.411.BJO-2023-0094.R1, 2023.
De Sousa, M. R., Luiz, A., and Ribeiro, P.: Revisión Sistemática y Metaanálisis de Estudios de Diagnóstico y Pronóstico: una Guía, Arq. Bras. Cardiol., 9, 235–245, 2009.
De Vecchi, E., Romanò, C. L., De Grandi, R., Cappelletti, L., Villa, F., and Drago, L.: Alpha defensin, leukocyte esterase, C-reactive protein, and leukocyte count in synovial fluid for pre-operative diagnosis of periprosthetic infection, Int. J. Immunopathol. Pharmacol., 2018, 32, https://doi.org/10.1177/2058738418806072, 2018.
Dijkman, C., Thomas, A. R., Koenraadt, K. L. M., Ermens, A. A. M., and van Geenen, R. C. I.: Synovial neutrophilic gelatinase-associated lipocalin in the diagnosis of periprosthetic joint infection after total knee arthroplasty, Arch. Orthop. Trauma Surg., 140, 941–947, https://doi.org/10.1007/s00402-020-03427-1, 2020.
Dilley, J. E., Seetharam, A., Meneghini, R. M., and Kheir, M. M.: Synovial Fluid Absolute Neutrophil Count and Neutrophil-To-Lymphocyte Ratio are not Superior to Polymorphonuclear Percentage in Detecting Periprosthetic Joint Infection, J. Arthroplast., 38, 146–151, https://doi.org/10.1016/j.arth.2022.07.005, 2023.
Diniz, S. E., Ribau, A., Vinha, A., Oliveira, J. C., Abreu, M. A., and Sousa, R.: Simple and inexpensive synovial fluid biomarkers for the diagnosis of prosthetic joint infection according to the new EBJIS definition, J. Bone Joint Infect., 8, 109–118, https://doi.org/10.5194/jbji-8-109-2023, 2023.
Dinneen, A., Guyot, A., Clements, J., and Bradley, N.: Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection, Bone Joint J., 95B, 554–557, https://doi.org/10.1302/0301-620X.95B4.30388, 2013.
Fink, B., Hoyka, M., Weissbarth, E., Schuster, P., and Berger, I.: The graphical representation of cell count representation: A new procedure for the diagnosis of periprosthetic joint infections, Antibiotics, 10, 346, https://doi.org/10.3390/antibiotics10040346, 2021.
Ghanem, E., Parvizi, J., Burnett, R. S. J., Sharkey, P. F., Keshavarzi, N., Aggarwal, A., and Barrack, R. L.: Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty, J. Bone Joint Surg., 90, 1637–1643, https://doi.org/10.2106/JBJS.G.00470, 2008a.
Ghanem, E., Houssock, C., Pulido, L., Han, S., Jaberi, F. M., and Parvizi, J.: Determining “True” Leukocytosis in Bloody Joint Aspiration, J. Arthroplast., 23, 182–187, https://doi.org/10.1016/j.arth.2007.08.016, 2008b.
Gramlich, Y., Schnetz, M., Ruckes, C., Kemmerer, M., Kremer, M., Hoffmann, R., and Klug, A.: The optimal diagnostic cut-off of WBC and PMN counts for joint aspiration in periprosthetic joint infection is 2479/µL and 67 %, respectively: ICM criteria thresholds are too high, Arch. Orthop. Trauma Surg., 143, 5229–5238, https://doi.org/10.1007/s00402-023-04822-0, 2023.
Heckmann, N. D., Nahhas, C. R., Yang, J., Della Valle, C. J., Yi, P. H., Culvern, C. N., Gerlinger, T. L., and Nam, D.: Saline lavage after a “dry tap” the differential is still usefu, Bone Joint J., 102, 138–144, 2020.
Heckmann, N. D., Wang, J. C., Liu, K. C., Won, P., Chung, B. C., Mayer, L. W., Longjohn, D. B., Oakes, D. A., Christ, A. B., and Lieberman, J. R.: Refining the Role of Routine Synovial Alpha-Defensin in Periprosthetic Joint Infection Following Total Knee Arthroplasty: An Analysis of Limitations, J. Arthroplast., 38, 2691–2697, https://doi.org/10.1016/j.arth.2023.05.095, 2023.
Higuera, C. A., Zmistowski, B., Malcom, T., Barsoum, W. K., Sporer, S. M., Mommsen, P., Kendoff, D., Valle, C. J. D., and Parvizi, J.: Synovial fluid cell count for diagnosis of chronic periprosthetic hip infection, J. Bone Joint Surg., 99, 753–759, https://doi.org/10.2106/JBJS.16.00123, 2017.
Huang, J., Wang, J., Qin, L., Zhu, B., Huang, W., and Hu, N.: Combination of Synovial Fluid IL-4 and Polymorphonuclear Cell Percentage Improves the Diagnostic Accuracy of Chronic Periprosthetic Joint Infection, Front. Surg., 9, 843187, https://doi.org/10.3389/fsurg.2022.843187, 2022.
Huard, M., Detrembleur, C., Poilvache, H., Pastor y Geels, I., Van Cauter, M., Driesen, R., Yombi, J. C., Neyt, J., and Cornu, O.: Alpha Defensin: A Diagnostic Accuracy Depending on the Infection Definition Used, J. Arthroplast., 35, 1355–1360, https://doi.org/10.1016/j.arth.2019.12.010, 2020.
Ivy, M. I., Sharma, K., Greenwood-Quaintance, K. E., Tande, A. J., Osmon, D. R., Berbari, E. F., Mandrekar, J., Beauchamp, C. P., Hanssen, A. D., Abdel, M. P., Lewallen, D. G., Perry, K., Block, D. R., Snyder, M. R., and Patel, R.: Synovial fluid α defensin has comparable accuracy to synovial fluid white blood cell count and polymorphonuclear percentage for periprosthetic joint infection diagnosi, Bone Joint J., 103, 1119–1126, https://doi.org/10.1302/0301-620X.103B6.BJJ-2020-1741.R1, 2021.
Karlidag, T., Luo, T. D., Gehrke, T., and Citak, M.: How Reliable Is the Absolute Synovial Polymorphonuclear Neutrophil Cell Count in Diagnosing Periprosthetic Joint Infection?, J. Arthroplast., 39, 1060–1068, https://doi.org/10.1016/j.arth.2023.10.038, 2024.
Kheir, M. M., Tan, T. L., Shohat, N., Foltz, C., and Parvizi, J.: Routine diagnostic tests for periprosthetic joint infection demonstrate a high false-negative rate and are influenced by the infecting organism, 100, 2057–2065, https://doi.org/10.2106/JBJS.17.01429, 2018.
Klemt, C., Tirumala, V., Smith, E. J., Xiong, L., and Kwon, Y. M.: Complete blood platelet and lymphocyte ratios increase diagnostic accuracy of periprosthetic joint infection following total hip arthroplasty, Arch. Orthop. Trauma Surg., 143, 1441–1449, https://doi.org/10.1007/s00402-021-04309-w, 2023.
Kuo, F. C., Lin, P. C., Yen, S. H., Tan, T. L., Wu, C. T., and Wang, J. W.: Which Minor Criteria is the Most Accurate Predictor for the Diagnosis of Hip and Knee Periprosthetic Joint Infection in the Asian Population?, J. Arthroplast., 37, 2076–2081, https://doi.org/10.1016/j.arth.2022.05.002, 2022.
Kwon, Y. M., Antoci, V., Leone, W. A., Tsai, T. Y., Dimitriou, D., and Liow, M. H. L.: Utility of Serum Inflammatory and Synovial Fluid Counts in the Diagnosis of Infection in Taper Corrosion of Dual Taper Modular Stems, J. Arthroplast., 31, 1997–2003, https://doi.org/10.1016/j.arth.2016.02.020, 2016.
Kwon, Y. M., Mahajan, J., Tirumala, V., Oganesyan, R., Yeo, I., and Klemt, C.: Sensitivity and Specificity of Serum and Synovial Fluid Markers in Diagnosis of Infection in Head-Neck Taper Corrosion of Metal-On-Polyethylene Total Hip Arthroplasty, J. Arthroplast., 35, 3737–3742, https://doi.org/10.1016/j.arth.2020.06.058, 2020.
Lazarides, A. L., Vovos, T. J., Reddy, G. B., Kildow, B. J., Wellman, S. S., Jiranek, W. A., and Seyler, T. M.: Traditional Laboratory Markers Hold Low Diagnostic Utility for Immunosuppressed Patients With Periprosthetic Joint Infections, J. Arthroplast., 34, 1441–1445, https://doi.org/10.1016/j.arth.2019.03.013, 2019.
Lazic, I., Prodinger, P., Stephan, M., Haug, A. T., Pohlig, F., Langer, S., von Eisenhart-Rothe, R., and Suren, C.: Synovial calprotectin is a reliable biomarker for periprosthetic joint infections in acute-phase inflammation – a prospective cohort study, Int. Orthop., 46, 1473–1479, https://doi.org/10.1007/s00264-022-05421-1, 2022a.
Lazic, I., Burdach, A., Pohlig, F., von Eisenhart-Rothe, R., and Suren, C.: Utility of synovial calprotectin lateral flow test to exclude chronic prosthetic joint infection in periprosthetic fractures: a prospective cohort study, Sci. Rep., 12, 18385, https://doi.org/10.1038/s41598-022-22892-9, 2022b.
Lenski, M. and Scherer, M. A.: Synovial IL-6 AS inflammatory marker in periprosthetic joint infections, J. Arthroplast., 29, 1105–1109, https://doi.org/10.1016/j.arth.2014.01.014, 2014.
Levent, A., Neufeld, M. E., Piakong, P., Lausmann, C., Gehrke, T., and Citak, M.: Which International Consensus Meeting Preoperative Minor Criteria is the Most Accurate Marker for the Diagnosis of Periprosthetic Joint Infection in Hip and Knee Arthroplasty?, J. Arthroplast., 36, 3728–3733, https://doi.org/10.1016/j.arth.2021.06.030, 2021.
Li, H., Li, R., Erlong, N., Chai, W., Hao, L. B., Xu, C., Fu, J., Chen, J., and Zhu, F.: It can be unnecessary to combine common synovial fluid analysis and alpha-defensin tests for periprosthetic joint infection diagnosis, BMC Musculoskelet. Disord., 24, 529, https://doi.org/10.1186/s12891-023-06594-5, 2023.
Majors, I. and Jagadale, V. S.: Serum interleukin 6 could be a valuable initial diagnostic tool in prosthetic knee joint infections, European J. Orthop. Surg. Traumatol., 29, 1781–1788, https://doi.org/10.1007/s00590-019-02519-y, 2019.
Mason, J. B., Fehring, T. K., Odum, S. M., Griffin, W. L., and Nussman, D. S.: The Value of White Blood Cell Counts before Revision Total Knee Arthroplasty, J. Arthroplast., 18, 1038–1043, https://doi.org/10.1016/S0883-5403(03)00448-0, 2003.
McInnes, M. D. F., Moher, D., Thombs, B. D., McGrath, T. A., Bossuyt, P. M., Clifford, T., Cohen, J. F., Deeks, J. J., Gatsonis, C., Hooft, L., Hunt, H. A., Hyde, C. J., Korevaar, D. A., Leeflang, M. M. G., Macaskill, P., Reitsma, J. B., Rodin, R., Rutjes, A. W. S., Salameh, J. P., Stevens, A., Takwoingi, Y., Tonelli, M., Weeks, L., Whiting, P., and Willis, B. H.: Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement, J. Am. Med. Assoc., 319, 388–396, https://doi.org/10.1001/JAMA.2017.19163, 2018.
Mcnally, M., Sousa, R., Wouthuyzen-Bakker, M., Chen, A. F., Soriano, A., Vogely, H. C., Clauss, M., Higuera, C. A., and Trebše, R.: The EBJIS definition of periprosthetic joint infection, Bone Joint J., 103, 18–25, https://doi.org/10.1302/0301-620X.103B1.BJJ-2020-1381.R1, 2021.
Nilsdotter-Augustinsson, Å., Briheim, G., Herder, A., Ljunghusen, O., Wahlström, O., and Öhman, L.: Inflammatory response in 85 patients with loosened hip prostheses: A prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening, Acta Orthop., 78, 629–639, https://doi.org/10.1080/17453670710014329, 2007.
Omar, M., Ettinger, M., Reichling, M., Petri, M., Guenther, D., Gehrke, T., Krettek, C., Mommsen, P., Surgeon, O., and Student, M.: Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty, Bone Joint J., 97, 173–176, https://doi.org/10.1302/0301-620X.97B2.34550, 2015.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., and Wilson, W. R.: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the infectious diseases Society of America, Clin. Infect. Dis., 56, 1–25, https://doi.org/10.1093/cid/cis803, 2013.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., McGuinness, L. A., Stewart, L. A., Thomas, J., Tricco, A. C., Welch, V. A., Whiting, P., and Moher, D.: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, Brit. Med. J., 29, 372, https://doi.org/10.1136/bmj.n71, 2021.
Parvizi, J. and Gehrke, T.: Definition of Periprosthetic Joint Infection, J. Arthroplast., 29, 1331, https://doi.org/10.1016/J.ARTH.2014.03.009, 2014.
Parvizi, J., Ghanem, E., Menashe, S., Barrack, R. L., and Bauer, T. W.: Periprosthetic infection: What are the diagnostic challenges?, J. Bone Joint Surg., 138–147, https://doi.org/10.2106/JBJS.F.00609, 2006.
Patel, V. V., Ernst, S. M. C., Rangarajan, R., Blout, C. K., Lee, B. K., and Itamura, J. M.: Validation of new shoulder periprosthetic joint infection criteria, J. Should. Elbow Surg., 30, S71–S76, https://doi.org/10.1016/j.jse.2021.04.009, 2021.
Premkumar, A., Kolin, D. A., Farley, K. X., Wilson, J. M., McLawhorn, A. S., Cross, M. B., and Sculco, P. K.: Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States, J. Arthroplast., 36, 1484–1489, https://doi.org/10.1016/j.arth.2020.12.005, 2021.
Qin, L., Hu, N., Li, X., Chen, Y., Wang, J., and Huang, W.: Evaluation of synovial fluid neutrophil CD64 index as a screening biomarker of prosthetic joint infection, Bone Joint J., 102, 463–469, https://doi.org/10.1302/0301-620X.102B4.BJJ-2019-1271.R1, 2020.
Qin, L., Wang, H., Zhao, C., Chen, C., Chen, H., Li, X., Wang, J., Hu, N., and Huang, W.: Serum and Synovial Biomarkers for Distinguishing Between Chronic Periprosthetic Joint Infections and Rheumatoid Arthritis: A Prospective Cohort Study, J. Arthroplast., 37, 342–346, https://doi.org/10.1016/j.arth.2021.09.009, 2022.
Ren, Y., Biedermann, L., Gwinner, C., Perka, C., and Kienzle, A.: Serum and Synovial Markers in Patients with Rheumatoid Arthritis and Periprosthetic Joint Infection, J. Pers. Med., 12, 810, https://doi.org/10.3390/jpm12050810, 2022.
Rupp, M., Lau, E., Kurtz, S. M., and Alt, V.: Projections of Primary TKA and THA in Germany From 2016 Through 2040, Clin. Orthop. Relat. Res., 478, 1622–1633, https://doi.org/10.1097/CORR.0000000000001214, 2020.
Salimy, M. S., Blackburn, A. Z., Katakam, A., Bedair, H. S., and Melnic, C. M.: Utility of Diagnostic Markers in Late Periprosthetic Joint Infection Workup for Total Knee Arthroplasty Patients Who Received Antibiotics 48 Hours Before Aspiration, J. Arthroplast., 38, 1854–1860, https://doi.org/10.1016/j.arth.2023.03.010, 2023.
Salimy, M. S., Humphrey, T. J., Egan, C. R., Alpaugh, K., Bedair, H. S., and Melnic, C. M.: Diagnostic Test Performances for Identifying Periprosthetic Joint Infection in Hip Hemiarthroplasty, J. Am. Acad. Orthop. Surg., 32, 447–455, https://doi.org/10.5435/JAAOS-D-23-00305, 2024.
Schiffner, E., Latz, D., Karbowski, A., Grassmann, J. P., Thelen, S., Windolf, J., Jungbluth, P., and Schneppendahl, J.: Loosening of total knee arthroplasty – always aseptic?, J. Clin. Orthop. Trauma, 11, S234–S238, https://doi.org/10.1016/j.jcot.2019.05.001, 2020.
Schünemann, H. J., Brennan, S., Akl, E. A., Hultcrantz, M., Alonso-Coello, P., Xia, J., Davoli, M., Rojas, M. X., Meerpohl, J. J., Flottorp, S., Guyatt, G., Mustafa, R. A., Langendam, M., and Dahm, P.: The development methods of official GRADE articles and requirements for claiming the use of GRADE – A statement by the GRADE guidance group, J. Clin. Epidemiol., 159, 79–84, https://doi.org/10.1016/J.JCLINEPI.2023.05.010, 2023.
Schwartz, A. J., Wetters, N., Moric, M., Berend, K. R., Lombardi, A. V., Gehrke, T., Kendoff, D., Sierra, R. J., Kassel, C., Berend, M. E., and Valle, C. J. D.: Diagnosis of periprosthetic joint infection after unicompartmental knee arthroplasty, J. Arthroplast., 27, 46–50, https://doi.org/10.1016/j.arth.2012.03.033, 2012.
Shah, R. P., Plummer, D. R., Moric, M., Sporer, S. M., Levine, B. R., and Della Valle, C. J.: Diagnosing Infection in the Setting of Periprosthetic Fractures, J. Arthroplast., 31, 140–143, https://doi.org/10.1016/j.arth.2015.08.045, 2016.
Shahi, A., Deirmengian, C., Higuera, C., Chen, A., Restrepo, C., Zmistowski, B., and Parvizi, J.: Premature Therapeutic Antimicrobial Treatments Can Compromise the Diagnosis of Late Periprosthetic Joint Infection, Clin. Orthop. Relat. Res., 473, 2244–2249, https://doi.org/10.1007/s11999-015-4142-z, 2015.
Shahi, A., Tan, T. L., Kheir, M. M., Tan, D. D., and Parvizi, J.: Diagnosing Periprosthetic Joint Infection: And the Winner Is?, J. Arthroplast., 32, S232–S235, https://doi.org/10.1016/j.arth.2017.06.005, 2017.
Sharma, K., Ivy, M., Block, D. R., Abdel, M. P., Hanssen, A. D., Beauchamp, C., Perry, K. I., Rosemark, C. L., Greenwood-Quaintance, K. E., Mandrekar, J., and Patel, R.: Comparative analysis of 23 synovial fluid biomarkers for hip and knee periprosthetic joint infection detection, J. Orthop. Res., 38, 2664–2674, https://doi.org/10.1002/jor.24766, 2020.
Shohat, N., Goswami, K., Fillingham, Y., Tan, T. L., Calkins, T., Della Valle, C. J., George, J., Higuera, C., and Parvizi, J.: Diagnosing Periprosthetic Joint Infection in Inflammatory Arthritis: Assumption Is the Enemy of True Understanding, J. Arthroplast., 33, 3561–3566, https://doi.org/10.1016/j.arth.2018.07.016, 2018.
Shohat, N., Bauer, T., Buttaro, M., Budhiparama, N., Cashman, J., Della Valle, C. J., Drago, L., Gehrke, T., Marcelino Gomes, L. S., Goswami, K., Hailer, N. P., Han, S. B., Higuera, C. A., Inaba, Y., Jenny, J.-Y., Kjaersgaard-Andersen, P., Lee, M., Llinás, A., Malizos, K., Mont, M. A., Jones, R. M., Parvizi, J., Peel, T., Rivero-Boschert, S., Segreti, J., Soriano, A., Sousa, R., Spangehl, M., Tan, T. L., Tikhilov, R., Tuncay, I., Winkler, H., Witso, E., Wouthuyzen-Bakker, M., Young, S., Zhang, X., Zhou, Y., and Zimmerli, W.: Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? Can the Same Criteria be Used for Both Joints?: Proceedings of International Consensus on Orthopedic Infections, J. Arthroplast., 34, S325–S327, https://doi.org/10.1016/j.arth.2018.09.045, 2019.
Sousa, R., Serrano, P., Gomes Dias, J., Oliveira, J. C., Oliveira, A., and Dias, J. G.: Improving the accuracy of synovial fluid analysis in the diagnosis of prosthetic joint infection with simple and inexpensive biomarkers, Bone Joint J., 99, 351–357, https://doi.org/0.1302/0301-620X.99B3.BJJ-2016-0684.R1, 2017.
Sousa, R., Ribau, A., Alfaro, P., Burch, M.-A., Ploegmakers, J., McNally, M., Clauss, M., Wouthuyzen-Bakker, M., and Soriano, A.: The European Bone and Joint Infection Society definition of periprosthetic joint infection is meaningful in clinical practice: a multicentric validation study with comparison with previous definitions, Acta Orthop., 94, 8–18, https://doi.org/10.2340/17453674.2023.5670, 2023.
Strahm, C., Zdravkovic, V., Egidy, C., and Jost, B.: Accuracy of synovial leukocyte and polymorphonuclear cell count in patients with shoulder prosthetic joint infection, J. Bone Joint Infect., 3, 245–248, https://doi.org/10.7150/jbji.29289, 2018.
Streck, L. E., Gaal, C., Forster, J., Konrads, C., von Hertzberg-Boelch, S. P., and Rueckl, K.: Defining a synovial fluid white blood cell count threshold to predict periprosthetic infection after shoulder arthroplasty, J. Clin. Med., 11, 50, https://doi.org/10.3390/jcm11010050, 2022a.
Streck, L. E., Forster, J., von Hertzberg-Boelch, S. P., Reichel, T., Rudert, M., and Rueckl, K.: The role of synovial fluid aspiration in shoulder joint infections, BMC Musculoskelet. Disord., 23, 390, https://doi.org/10.1186/s12891-022-05285-x, 2022b.
Su, X., Zhu, B., Qin, L., Yang, J., Wei, L., Xu, Z., Wei, K., Wang, J., Chen, L., Zhao, C., Chen, C., Huang, W., Xiong, Y., and Hu, N.: Joint fluid interleukin-6 combined with the neutral polymorphonuclear leukocyte ratio (PMN %) as a diagnostic index for chronic periprosthesis infection after arthroplasty, J. Orthop. Traumatol., 24, 34, https://doi.org/10.1186/s10195-023-00712-8, 2023.
Tahta, M., Simsek, M. E., Isik, C., Akkaya, M., Gursoy, S., and Bozkurt, M.: Does inflammatory joint diseases affect the accuracy of infection biomarkers in patients with periprosthetic joint infections? A prospective comparative reliability study, J. Orthop. Sci., 24, 286–289, https://doi.org/10.1016/j.jos.2018.08.022, 2019.
Theil, C., Ackmann, T., Gosheger, G., Puetzler, J., Moellenbeck, B., Schwarze, J., Schulze, M., and Klingebiel, S.: Synovial fluid pH is as specific as synovial leukocyte count but less sensitive for the diagnosis of chronic prosthetic joint infection, J. Orthop. Traumatol., 23, 52, https://doi.org/10.1186/s10195-022-00672-5, 2022.
Theil, C., Moellenbeck, B., Schwarze, J., Puetzler, J., Klingebiel, S., Bockholt, S., and Gosheger, G.: Can the Current Thresholds for Synovial Cell Count and Neutrophil Percentage to Diagnose Prosthetic Joint Infection be Applied to Metal-on-Metal Rotating Hinge Total Knee Arthroplasty?, J. Arthroplast., 39, 801–805, https://doi.org/10.1016/j.arth.2023.08.073, 2024.
Thiesen, D. M., Koniker, A., Gehrke, T., Linke, P., Ohlmeier, M., Salber, J., and Citak, M.: The Impact of α-Defensin Test in Diagnosing Periprosthetic Infection After Total Ankle Arthroplasty, J. Foot Ankle Surg., 58, 1125–1128, https://doi.org/10.1053/j.jfas.2019.03.006, 2019.
Trampuz, A., Hanssen, A. D., Osmon, D. R., Mandrekar, J., Steckelberg, J. M., and Patel, R.: Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection, Am. J. Med., 117, 556–562, https://doi.org/10.1016/j.amjmed.2004.06.022, 2004.
Trampuz, A., Piper, K. E., Jacobson, M. J., Hanssen, A. D., Unni, K. K., Osmon, D. R., Mandrekar, J. N., Cockerill, F. R., Steckelberg, J. M., Greenleaf, J. F., and Patel, R.: Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection, N. Engl. J. Med., 357, 654–663, https://doi.org/10.1056/NEJMoa061588, 2007.
van den Kieboom, J., Tirumala, V., Xiong, L., Klemt, C., and Kwon, Y. M.: Concomitant Hip and Knee Periprosthetic Joint Infection in Periprosthetic Fracture: Diagnostic Utility of Serum and Synovial Fluid Markers, J. Arthroplast., 36, 722–727, https://doi.org/10.1016/j.arth.2020.08.029, 2021.
Wang, H., Qin, L., Wang, J., Hu, N., and Huang, W.: Combined serum and synovial C-reactive protein tests: a valuable adjunct to the diagnosis of chronic prosthetic joint infection, BMC Musculoskelet. Disord., 22, 670, https://doi.org/10.1186/s12891-021-04545-6, 2021a.
Wang, H., Qin, L., Wang, J., and Huang, W.: Synovial fluid IL-1β appears useful for the diagnosis of chronic periprosthetic joint infection, J. Orthop. Surg. Res., 16, 144, https://doi.org/10.1186/s13018-021-02296-7, 2021b.
Wang, Y., Li, G., Ji, B., Xu, B., Zhang, X., Maimaitiyiming, A., and Cao, L.: Diagnosis of periprosthetic joint infections in patients who have rheumatoid arthritis application of routine serological and synovial fluid indexes, Bone Joint Res., 12, 559–570, https://doi.org/10.1302/2046-3758.129.BJR-2022-0432.R1, 2023.
Whiting, P. F., Rutjes, A. W. S., Westwood, M. E., Mallett, S., Deeks, J. J., Reitsma, J. B., Leeflang, M. M. G., Sterne, J. A. C., and Bossuyt, P. M. M.: Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies, Ann. Intern Med., 155, 529–536, https://doi.org/10.7326/0003-4819-155-8-201110180-00009, 2011.
Workgroup Convened by the Musculoskeletal Infection Society: New Definition for Periprosthetic Joint Infection, J. Arthroplast., 26, 1136–1138, https://doi.org/10.1016/j.arth.2011.09.026, 2011.
Yi, P. H., Cross, M. B., Moric, M., Levine, B. R., Sporer, S. M., Paprosky, W. G., Jacobs, J. J., and Della Valle, C. J.: Do Serologic and Synovial Tests Help Diagnose Infection in Revision Hip Arthroplasty With Metal-on-metal Bearings or Corrosion?, Clin. Orthop. Relat. Res., 473, 498–505, https://doi.org/10.1007/s11999-014-3902-5, 2015.
Zahar, A., Lausmann, C., Cavalheiro, C., Dhamangaonkar, A. C., Bonanzinga, T., Gehrke, T., and Citak, M.: How Reliable Is the Cell Count Analysis in the Diagnosis of Prosthetic Joint Infection?, J. Arthroplast., 33, 3257–3262, https://doi.org/10.1016/j.arth.2018.05.018, 2018.
Zhang, Z., Cai, Y., Bai, G., Zhang, C., Li, W., Yang, B., and Zhang, W.: The value of calprotectin in synovial fluid for the diagnosis of chronic prosthetic joint infection Strengths and limitations, Bone Joint Res., 9, 450–457, https://doi.org/10.1302/2046-3758.98.BJR-2019-0329.R2, 2020.
Zmistowski, B., Restrepo, C., Huang, R., Hozack, W. J., and Parvizi, J.: Periprosthetic Joint Infection Diagnosis. A Complete Understanding of White Blood Cell Count and Differential, J. Arthroplast., 27, 1589–1593, https://doi.org/10.1016/j.arth.2012.03.059, 2012.