the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Glenohumeral joint septic arthritis and osteomyelitis caused by Moraxella catarrhalis after arthroscopic rotator cuff repair: case report and literature review
Yong-Beom Kim
Jinjae Kim
Min Gon Song
Tae Hyong Kim
Tae-Yoon Choi
Gi-Won Seo
Moraxella catarrhalis commonly colonizes the upper respiratory tract of humans, but infection caused by M. catarrhalis after orthopedic surgery is rare. Here, we report the first case of septic arthritis of the shoulder caused by an M. catarrhalis infection and outline the diagnosis and treatment steps as well as differences compared with other cases.
- Article
(2420 KB) - Full-text XML
- BibTeX
- EndNote
Moraxella catarrhalis is an aerobic, nonmotile, Gram-negative diplococcus that commonly colonizes the upper respiratory tract of humans. It is uncommonly associated with invasive disease unless the patient is in an immunocompromised state. Common clinical manifestation are limited to otitis media and respiratory tract infections, such as sinusitis, bronchitis, and pneumonia (Verduin et al., 2002). Septic arthritis, vertebral osteomyelitis, and diskitis caused by M. catarrhalis without concomitant bacteremia have rarely been reported (Brunckhorst et al., 2020; Craig and Wehrle, 1983; Evangelista et al., 2017; Izraeli et al., 1989; Leonardou et al., 2005; Melendez and Johnson, 1991; Olivieri et al., 2004; Prallet et al., 1991; Minoza et al., 2015). In septic arthritis, prompt diagnosis and treatment with antibiotics and surgery are crucial to prevent further joint damage and ensure a good prognosis (Shirtliff and Mader, 2002). Septic arthritis of the glenohumeral joint caused by M. catarrhalis has not yet been reported. We present the case of a patient who developed septic arthritis of the right glenohumeral joint caused by M. catarrhalis after arthroscopic rotator cuff repair. In addition, we review the literature in the field of orthopedics for case reports of septic arthritis caused by M. catarrhalis.
The patient provided written informed consent for the publication of this report and the accompanying images.
2.1 Clinical presentation
A 71-year-old patient with hypertension and hyperlipidemia presented with a 2-year history of right shoulder pain. The patient was diagnosed with a rotator cuff tear and impingement syndrome at another hospital and received acupuncture and prolotherapy until October 2022 at a local clinic. Their symptoms worsened after they had a fall on 24 December 2022. The patient received local steroid injections in early January 2023, but the symptoms did not improve. On 12 January 2023, the patient underwent arthroscopic rotator cuff repair and acromioplasty at another hospital. On postoperative day (POD) 5, they developed a mild fever and swelling, a burning sensation, and redness of the right shoulder. The patient was closely monitored via regular follow-ups, but no discharge was noted. Laboratory parameters – such as white blood cell (WBC) count (10 000 c µL−1 vs. normal range of 4000–10 000 c µL−1) and C-reactive protein (CRP) level (9.7 mg dL−1 vs. normal range of 0.0–0.5 mg dL−1) – were elevated. Cefazolin administration was started on POD 5. Despite receiving cefazolin until POD 7, there was no improvement in the symptoms. Therefore, the patient was transferred to our hospital. At admission, the patient had no fever, and laboratory analysis showed that their WBC count was 8500 c µL−1 and CRP level was 7.28 mg dL−1.
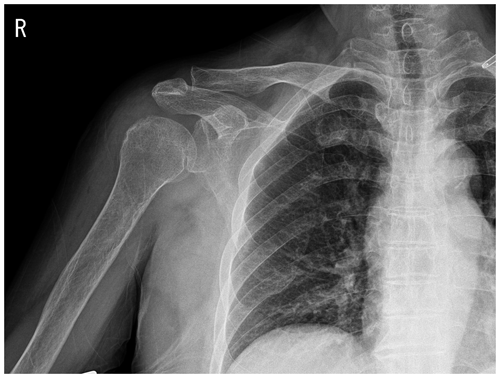
Figure 1Plain radiograph showing osteoarthritic changes in the right acromioclavicular joint and soft-tissue swelling around the right shoulder.
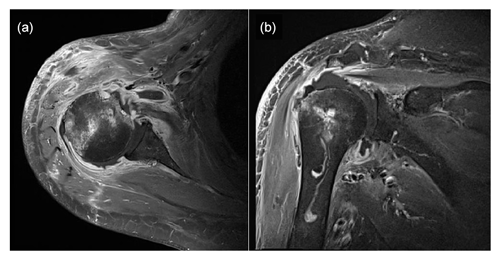
Figure 2Enhanced magnetic resonance images showing effusion in the right glenohumeral joint with synovial hypertrophy and combined diffuse heterogeneous enhancement along the right glenohumeral joint capsule. Additionally, an ill-defined bone marrow signal intensity change is observed in the right humeral head. Panel (a) presents a T1-weighted, contrast-enhanced fat suppression axial-view image, whereas panel (b) shows a T1-weighted, contrast-enhanced fat suppression coronal-view image.
2.2 Imaging studies
Plain-film radiographs of the right shoulder showed osteoarthritic changes in the right acromioclavicular joint and soft-tissue swelling around the right shoulder (Fig. 1). Enhanced magnetic resonance imaging (MRI) showed effusion in the right glenohumeral joint with synovial hypertrophy and combined diffuse heterogeneous enhancement along the right glenohumeral joint capsule. In addition, an ill-defined change in the bone marrow signal intensity in the right humeral head was observed (Fig. 2). After full radiologic evaluation, multidisciplinary discussion was led by an orthopedic surgeon, an infectious disease specialist, a radiologist, and a clinical microbiologist. After team discussion, postoperative septic arthritis was suspected, and diagnostic and therapeutic arthroscopic surgery was scheduled. All previous antimicrobial agents were discontinued to improve the diagnostic yield of the causative pathogen in resection tissue culture. Given the know microbial epidemiology of postsurgical infection, the initial empirical antimicrobial agents after arthroscopic surgery should cover common Gram-positive skin flora as well as Gram-negative bacteria due to the patient's age.
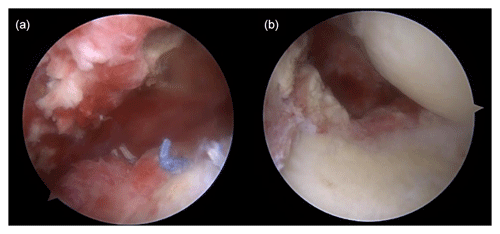
Figure 3Arthroscopic findings showing (a) severe synovitis with pus-like fluid on the intra-articular side and (b) inflammatory granulation tissue and severe bursitis on the bursal side.
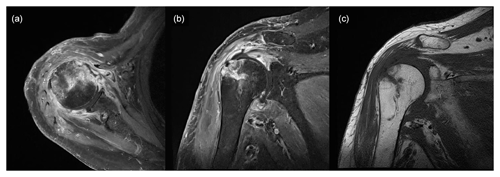
Figure 4Magnetic resonance image obtained 3 weeks after surgery showing increased signal intensity in the cortex and medulla of the humeral head, while the same areas appear well-demarcated and darker in the non-enhanced image: (a) T1-weighted, contrast-enhanced fat suppression axial-view image; (b) T1-weighted, contrast-enhanced fat suppression coronal-view image; (c) T1-weighted, non-contrast-enhanced coronal-view image.
2.3 Treatment
Shoulder arthroscopy was performed on 20 January 2023 for irrigation, and specimens were obtained via debridement. The arthroscopic findings of the intra-articular side were severe synovitis with pus-like fluid, whereas those of the bursal side were inflammatory granulation tissue and severe bursitis with a well-maintained previously repaired rotator cuff (Fig. 3). Gram staining and pathology tests were performed on the specimen. The patient remained admitted postoperatively and was administered empirical intravenous vancomycin (2 g d−1) and cefepime (6 g d−1) while awaiting culture results until POD 5. Gram staining showed Gram-negative diplococcus, and subsequent tissue culture revealed M. catarrhalis. Based on pathological examination, the specimen turned out to be acute and chronic nonspecific inflammation with granulation tissue formation. Antimicrobial agents were de-escalated to vancomycin and ampicillin/sulbactam (3 g d−1) until POD 20. The vancomycin targeting potential Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) was not initially discontinued, even after confirmation of M. catarrhalis as the causative pathogen, because this bacteria was not expected to cause such surgical infection as commonly. MRI performed 3 weeks after surgery revealed that the bone marrow signal intensity of the humeral head had increased on a contrast-enhanced, T1-weighted image, and the marrow of the humeral head appeared darker and well-demarcated on a non-enhanced, T1-weighted image (Fig. 4). Concurrent osteomyelitis of humeral head was later diagnosed, and antimicrobial agents were administered for 6 weeks after surgery.
After 3 weeks of antibiotic administration, the patient had a systemic allergic rash; therefore, the antibiotics were changed to intravenous ceftriaxone alone (2 g d−1). The medical treatment was then simplified to target only M. catarrhalis due to an unavoidable adverse drug event. Despite this, the systemic allergic rash persisted; therefore, the medication was changed to oral moxifloxacin (400 mg d−1). The rash seemed to improve for a while; however, 2 d after changing the antibiotic, the rash worsened again, so medication was changed to oral doxycycline (200 mg d−1). After changing antibiotics, allergy symptoms improved and treatment was maintained until 6 weeks after surgery.
Laboratory analysis performed after 6 weeks of antibiotic administration revealed normalized WBC counts and CRP levels of 7100 c µL−1 and 0.17 mg dL−1, respectively. A total of 11 weeks after surgery, the patient reported a full range of motion in the right shoulder joint, with no significant limitations with respect to daily activities. Additionally, no notable symptoms were observed.
Septic arthritis is an unfortunate healthcare-associated complication of arthroscopy, with an overall estimated incidence of less than 1 % (Bauer et al., 2015). It is a potentially serious and debilitating condition that can occur when bacteria or other microorganisms infect a joint. Surgery, underlying joint disease, diabetes mellitus, presence of prosthesis, skin defect or infection, advanced age, and immunosuppressive medication are known risk factors of septic arthritis (García-Arias et al., 2011). Moreover, invasive procedures (similar to what the patient experienced in this case) could be a risk factor for septic arthritis; intra-articular steroid injections which can cause septic arthritis in 4 out of 10 000 cases (Geirsson et al., 2008) and various cases of septic arthritis associated with acupuncture have been reported (Woo et al., 2009).
The most common pathogens associated with shoulder infection after shoulder arthroscopy are Cutibacterium acnes and staphylococci (Bauer et al., 2015; Kumar and Thilak, 2016). Other pathogens that can cause shoulder infection include Pseudomonas aeruginosa, Mycobacterium tuberculosis, and Actinomyces (Aydin et al., 2014; Bauer et al., 2015; Khan et al., 2017; Pauzenberger et al., 2017). Here, we report the first case of septic arthritis of the shoulder caused by M. catarrhalis infection.
Moraxella is a genus of opportunistic pathogens that infect various body parts, including the respiratory tract, middle ear, and sinuses (Verduin et al., 2002). Moraxella is commonly associated with respiratory tract infections, often leading to conditions such as pneumonia. Typically, chest X-rays reveal a bronchopneumonia pattern, while computed tomography (CT) scans demonstrate bronchial wall thickening, bilateral distribution, and segmental patterns (Hirai et al., 2020). However, we present a case of Moraxella infection in which the patient did not exhibit respiratory symptoms; moreover, chest X-ray findings were unremarkable. It is known that Moraxella can cause meningitis and eye infections other than respiratory infections, but bone and joint infections have rarely been reported, and they have been described mostly in adults, particularly in those with underlying joint disease or immunodeficiency (Murphy and Parameswaran, 2009). Additionally, there are rare cases in which a pathogen has been reported as the causative bacteria of prosthetic joint infection. Two of the three prosthetic joint infection cases were immunocompromised patients (Evangelista et al., 2017; Minoza et al., 2015; Leonardou et al., 2005).
Table 1Reported cases of infectious arthritis with Moraxella catarrhalis.

n/a: not applicable. q4: administration every 4 h.
We reviewed five published cases of septic arthritis on joints other than glenohumeral joints caused by M. catarrhalis (Table 1) (Craig and Wehrle, 1983; Izraeli et al., 1989; Melendez and Johnson, 1991; Olivieri et al., 2004; Rogers, 2023). One patient had an immunodeficiency as well as underlying joint disease, one other patient had an immunodeficiency with no joint disease, and three patients had no underlying joint disease or immunodeficiency. In three patients, surgical interventions such as arthrotomy and drainage, open drainage, and open debridement were performed along with antibiotic treatment; the remaining two patients only received antibiotic treatment and did not undergo surgery. Antibiotics, such as levofloxacin, clindamycin, ceftriaxone, and gentamicin, were administered for at least 2 weeks in five patients. Two patients reported by Craig and Wehrle (1983) and Melendez and Johnson (1991) received penicillin as their initial treatment.
The differences between our patient and those in previously published case reports are as follows: our patient had an underlying joint disease with no immunodeficiency. This is the first reported case of septic arthritis of the glenohumeral joint occurring after arthroscopic surgery. Our patient's septic arthritis progressed to osteomyelitis. After surgery, vancomycin and beta-lactams were administered to treat osteomyelitis; however, side effects (such as skin rash and itching) occurred, and the treatment was changed to cephalosporin antibiotics and doxycycline.
The clinical presentation of septic arthritis caused by M. catarrhalis is similar to that of other types of septic arthritis, with symptoms such as joint pain, swelling, stiffness, fever, and chills. However, diagnosis can be challenging because M. catarrhalis is not commonly associated with joint infection, and other causes of joint inflammation must be ruled out. Prompt and aggressive antibiotic therapy is necessary to control the infection and prevent further joint damage. M. catarrhalis is generally susceptible to beta-lactams, tetracycline, quinolones, and aminoglycosides. However, most M. catarrhalis isolates produce inducible beta-lactamase (Vaneechoutte et al., 2015). Surgical intervention such as early arthroscopic joint irrigation or debridement may be necessary in some cases. In conclusion, although M. catarrhalis is not a common cause of septic arthritis, it should be considered in the differential diagnosis of joint infection, particularly in adults with underlying joint diseases or immunodeficiency. Early diagnosis and appropriate antibiotic therapy are essential for achieving favorable outcomes and preventing long-term joint damage.
Infection caused by M. catarrhalis is rare after orthopedic surgery, and our study is the first to report septic arthritis of the glenohumeral joint. Although the clinical symptoms are similar to those of septic arthritis caused by commonly encountered bacteria, it is important to test for antibiotic susceptibility using culture. Joint damage should be minimized through appropriate antibiotic therapy and surgical intervention.
The patient data generated and/or analyzed during the current study are presented in Figs. 1–4. Data from other existing publications discussed in this work are listed in Table 1.
YBK and MGS: conceptualization; JK and MGS: data curation; GWS: formal analysis; TYC: methodology; JK: writing – original draft; GWS, YBK, and THK: writing – review and editing. All authors have read and approved the manuscript.
The contact author has declared that none of the authors has any competing interests.
The patient provided written informed consent for the publication of this report and the accompanying images.
Funding bodies did not play a role in the collection, analysis, or interpretation of data; they also did not contribute to the writing of this manuscript. The funding bodies supported the cost of correcting the English in this paper.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We would like to thank Editage (http://www.editage.co.kr, last access: 19 March 2023) for English language editing of a draft of the manuscript.
This work was supported by the Soonchunhyang University Research Fund.
This paper was edited by Parham Sendi and reviewed by two anonymous referees.
Aydin, N., Sirin, E., Aydemir, A. N., and Zengin, G.: Pseudomonas osteomyelitis of the proximal humerus after arthroscopic rotator cuff repair, Acta Orthop. Traumatol. Turc., 48, 685–689, https://doi.org/10.3944/AOTT.2014.13.0015, 2014.
Bauer, T., Boisrenoult, P., and Jenny, J. Y.: Post-arthroscopy septic arthritis: Current data and practical recommendations, Orthop. Traumatol. Surg. Res., 101, S347–350, https://doi.org/10.1016/j.otsr.2015.09.004, 2015.
Brunckhorst, T., Toombes, S., and Beale, M.: Moraxella catarrhalis discitis: the first reported case, Intern. Med. J., 50, 381–382, https://doi.org/10.1111/imj.14758, 2020.
Craig, D. B. and Wehrle, P. A.: Branhamella catarrhalis septic arthritis, J. Rheumatol., 10, 985–986, 1983.
Evangelista, P., Evangelista, G., and Ogrich, L.: Infection after Total Knee Arthroplasty by M. catarrhalis Associated with Rheumatoid Arthritis and DMARDs Therapy, Bull. Hosp. Jt. Dis., 75, 140–142, 2017.
Garcia-Arias, M., Balsa, A., and Mola, E. M.: Septic arthritis, Best Pract. Res. Clin. Rheumatol., 25, 407–421, https://doi.org/10.1016/j.berh.2011.02.001, 2011.
Geirsson, A. J., Statkevicius, S., and Vikingsson, A.: Septic arthritis in Iceland 1990-2002: increasing incidence due to iatrogenic infections, Ann. Rheum. Dis., 67, 638–643, https://doi.org/10.1136/ard.2007.077131, 2008.
Hirai, J., Kinjo, T., Koga, T., Haranaga, S., Motonaga, E., and Fujita, J.: Clinical characteristics of community-acquired pneumonia due to Moraxella catarrhalis in adults: a retrospective single-centre study, BMC Infect. Dis., 20, 821, https://doi.org/10.1186/s12879-020-05564-9, 2020.
Izraeli, S., Flasterstein, B., Shamir, R., Rachmel, A., Nitzan, M., Drucker, M., and Samra, Z.: Branhamella catarrhalis as a cause of suppurative arthritis, Pediatr. Infect. Dis. J., 8, 256–257, 1989.
Khan, P. S., Thilak, J., George, M. J., Nair, A. V., and Madanan, A.: Tubercular infection after arthroscopic rotator cuff repair, Knee Surg. Sports Traumatol. Arthrosc., 25, 2205–2207, https://doi.org/10.1007/s00167-015-3968-y, 2017.
Kumar, M. and Thilak, J.: Infected shoulder joint with loose Suture Anchor in the joint after Bankart's Repair- A Case Report, J. Orthop. Case Rep., 6, 6–8, 2016.
Leonardou, A., Giali, S., Daoussis, D., Siambi, V., Gogos, H., and Liossis, S. N.: Moraxella catarrhalis-induced septic arthritis of a prosthetic knee joint in a patient with rheumatoid arthritis treated with anakinra: comment on the article by Schiff et al., Arthritis Rheum., 52, 1337; author reply 1338, https://doi.org/10.1002/art.21003, 2005.
Melendez, P. R. and Johnson, R. H.: Bacteremia and septic arthritis caused by Moraxella catarrhalis, Rev. Infect. Dis., 13, 428–429, https://doi.org/10.1093/clinids/13.3.428, 1991.
Minoza, A., Boutoille, D., Aubin, G. G., Hauet, P., Grossi, O., Touchais, S., Bemer, P., and Corvec, S.: Report of a Moraxella catarrhalis prosthetic joint infection in an immunocompetent woman and review of the literature of haematogenous infection due to throat flora bacteria, JMM Case Reports, 2, https://doi.org/10.1099/jmmcr.0.000053, 2015.
Murphy, T. F. and Parameswaran, G. I.: Moraxella catarrhalis, a human respiratory tract pathogen, Clin. Infect. Dis., 49, 124–131, https://doi.org/10.1086/599375, 2009.
Olivieri, I., Padula, A., Armignacco, L., Sabatella, V., and Mancino, M.: Septic arthritis caused by Moraxella catarrhalis associated with infliximab treatment in a patient with undifferentiated spondarthritis, Ann. Rheum. Dis., 63, 105–106, https://doi.org/10.1136/ard.2003.006270, 2004.
Pauzenberger, L., Grieb, A., Hexel, M., Laky, B., Anderl, W., and Heuberer, P.: Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis, Knee Surg. Sports Traumatol. Arthrosc., 25, 595–601, https://doi.org/10.1007/s00167-016-4202-2, 2017.
Prallet, B., Lucht, F., and Alexandre, C.: Vertebral osteomyelitis due to Branhamella catarrhalis., Rev. Infect. Dis., 13, 769, https://doi.org/10.1093/clinids/13.4.769, 1991.
Rogers, N. G.: Moraxella catarrhalis Septic Arthritis Unveils Undiagnosed Systemic Lupus Erythematous in a Pediatric Patient, Cureus, 15, e50909, https://doi.org/10.7759/cureus.50909, 2023.
Shirtliff, M. E. and Mader, J. T.: Acute septic arthritis, Clin. Microbiol. Rev., 15, 527–544, https://doi.org/10.1128/CMR.15.4.527-544.2002, 2002.
Vaneechoutte, M., Nemec, A., Kampfer, P., Cools, P., and Wauters, G.: Acinetobacter, Chryseobacterium, Moraxella, and Other Nonfermentative Gram-Negative Rods, in: Manual of Clinical Microbiology, 11th edn., edited by: James, H. J., Karen, C. C., Guido, F., Michael, A. P., Marie, L. L., Sandra, S. R., and David, W. W., 813–837, https://doi.org/10.1128/9781555816728.ch42, 2015.
Verduin, C. M., Hol, C., Fleer, A., van Dijk, H., and van Belkum, A.: Moraxella catarrhalis: from emerging to established pathogen, Clin. Microbiol. Rev., 15, 125–144, https://doi.org/10.1128/CMR.15.1.125-144.2002, 2002.
Woo, P. C., Lau, S. K., and Yuen, K. Y.: First report of methicillin-resistant Staphylococcus aureus septic arthritis complicating acupuncture: simple procedure resulting in most devastating outcome, Diagn. Microbiol. Infect. Dis., 63, 92–95, https://doi.org/10.1016/j.diagmicrobio.2008.08.023, 2009.




