the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Mycetoma caused by Madurella mycetomatis in immunocompromised patients – a case report and systematic literature review
Lotje A. Hoogervorst
Lindsey S. op de Coul
Arghya Ray
Pieter Bas de Witte
Mark G. J. de Boer
The aim of this study was to review the available literature concerning Madura foot (“mycetoma”) caused by Madurella mycetomatis in immunocompromised patients. With a systematic literature search, we identified only three papers, describing a total of three immunocompromised patients. Hence, the clinical presentation and prognosis of the disease in this patient population have not yet been well described. In addition, we present a case from our institution, illustrating the complexity of the treatment of this rare disease. Although very rare in non-endemic countries, we emphasize that mycetoma should be included in the differential diagnoses of (immunocompromised) patients who have been residing in a geographical area where the disease is endemic and presenting with soft tissue inflammation of one of the extremities.
- Article
(746 KB) - Full-text XML
-
Supplement
(564 KB) - BibTeX
- EndNote
A Madura foot (“mycetoma”) is a chronic granulomatous soft tissue infection, with or without concomitant bone involvement, of the foot (El Muttardi et al., 2010; van de Sande, 2013). It has been categorized as a neglected tropical disease by the World Health Organization (WHO) (World Health Organization, 2019; Verma and Jha, 2019), and the exact prevalence and incidence of mycetoma are unclear (van de Sande, 2013). The infection is endemic in certain equatorial regions: Latin America (e.g., Venezuela), Africa (e.g., Somalia), Yemen, and India (van de Sande, 2013; World Health Organization, 2019). A mycetoma is caused by infection with specific filamentous bacteria (actinomycetoma) or fungi (eumycetoma) (van de Sande, 2013; Verma and Jha, 2019; Welsh et al., 2007). Eumycetoma is often caused by Madurella mycetomatis, which is a fungus found in soil (van de Sande, 2013; Welsh et al., 2007). A Madurella mycetomatis infection is generally acquired following inoculation of the fungus into the skin while walking barefooted on contaminated soil (World Health Organization, 2019; Welsh et al., 2007). Once it has entered the body, the fungus will grow and capture itself in small granules (i.e., grains) (van de Sande, 2013). From these grains, the fungal infection slowly progresses over months or even years into larger subcutaneous masses that will eventually invade bone tissue (van de Sande, 2013).
Many studies describing mycetoma infections in immunocompetent patients have been published (Fasciana et al., 2018; Karrakchou et al., 2020; Asly et al., 2010; Brufman et al., 2015; Mestre et al., 2015; Sigera et al., 2020; Fida et al., 2018; Elmaataoui et al., 2011; Sampaio et al., 2015). However, studies reporting on mycetoma infections in immunocompromised patients are rare. In this paper we will review the currently available literature with regard to Madura foot caused by the fungus Madurella mycetomatis in immunocompromised patients, and we describe a case of a kidney transplant recipient presenting with this disease.
A 53-year-old man presented for the first time to our orthopedic outpatient clinic with a then 3-year history of soft tissue swellings on his right hallux which had slowly increased in volume. The patient had been living in the Netherlands for 23 years and regularly visited his native country (Somalia). His medical history included type-2 diabetes mellitus, treatment for latent tuberculosis and a failed donor kidney transplantation 1 year earlier due to polycystic kidney disease. At the time of presentation, the patient was on prednisolone, tacrolimus and mycophenolate mofetil. The lesions on his foot were painless and did not disturb his daily and routine activities. The patient sought medical care due to recently developed small skin lesions which produced minimal amounts of fluid. Physical examination revealed two palpable, movable, soft tissue swellings (approximately 3 by 3 cm) and some fistulas along the medial and plantar surfaces of the right hallux. The lesions were not painful when applying pressure and did not compromise the range of motion of the hallux.
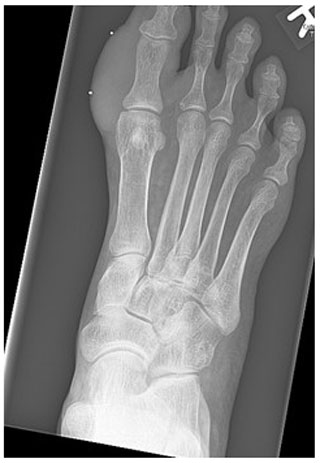
Figure 1Radiograph of the right foot showing the soft tissue swelling on the medial side of the proximal phalanx of the right hallux.
2.1 Radiology
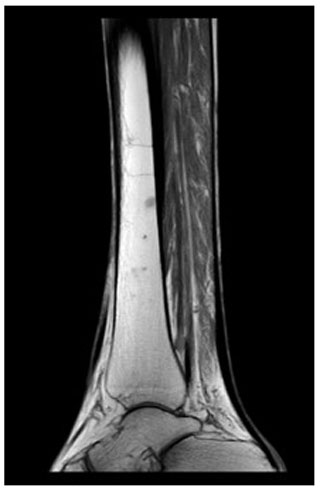
Radiographs revealed soft tissue swelling on the medial side of the right hallux without signs of bone destruction (Fig. 1). Magnetic resonance imaging (MRI) was performed to detect possible osteomyelitis. The MRI showed multiple noduli with “dot-in-circle signs” around the hallux extending into the first web space (largest nodule: 3.8 by 3.9 by 3.3 cm) and multiple intra-osseous lesions in the distal tibia (Figs. 2 and 3). No (cortical) bone destruction, bone marrow edema, or signs of osteomyelitis were observed. Ultrasound-guided biopsy was performed to obtain tissue samples, which were analyzed for bacterial and mycological pathogens as well as for histological assessment.
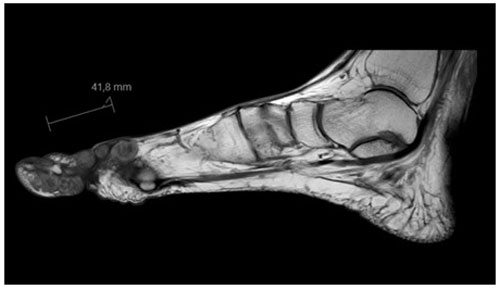
Figure 2MRI of the foot (sagittal view) showing multiple subcutaneous swellings with “dot-in-circle signs” around the hallux extending into the first web space (the largest nodule measured 3.8 by 3.9 by 3.3 cm).
2.2 Pathology and microbiology
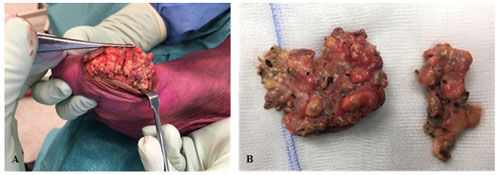
Histopathological assessment revealed fungal hyphae, plasma cells, neutrophils, lymphocytes and macrophages. Fungal culture test identified the fungus Madurella mycetomatis after 22 d of incubation, confirming the diagnosis of Madura foot. Shared decision making resulted in antifungal therapy and macroscopical wide-local surgical excision of the lesions (Fig. 4).
2.3 Treatment and clinical course
Itraconazole emulsion 200 mg three times daily for a 3 d period followed by 200 mg two times daily was prescribed immediately post-operation. Due to the itraconazole–tacrolimus drug interaction, the tacrolimus dose was reduced from 1 to 0.5 mg on alternate days with through levels fluctuating between 3 and 6 µg L−1. Mycophenolate was continued with exposure slightly under the target range (area under the curve – AUC – 19 mg h L−1). The itraconazole serum concentration was, measured after 7 d of usage, 1.11 mcg mL−1, indicating an acceptable therapeutic level (a minimal inhibitory concentration (MIC) of 0.125–0.06 mcg mL−1 and a through concentration of >1.0 mcg mL−1 for effective treatment). One month later, due to side effects (nausea and diarrhea), the itraconazole was switched to voriconazole 200 mg orally twice a day. Again, after using voriconazole for 1 month, side effects occurred (dizziness and visual disturbances). Hence, the voriconazole was switched to posaconazole 300 mg oral capsules once a day. The posaconazole serum concentration reached, measured after 9 d of usage, acceptable therapeutic levels (2.43 mg L−1) (a MIC of 0.06–0.06 µg L−1 and a through concentration of >1.25 mg L−1 for salvage therapy).
Despite surgical excision and antifungal therapy, recurrence of the Madura foot was confirmed after 6 months. Amputation of the forefoot was considered. However, after multidisciplinary deliberation, treatment was commenced with surgical re-excision followed by posaconazole 300 mg oral capsules once daily. Madurella mycetomatis was again identified in the resected tissue. The patient was monitored at the infectious disease outpatient clinic with a 2-month interval. The infection appeared to be maximally suppressed; there were no clinical signs of recurrence, and MRI (undertaken at 6-month intervals) did not show any signs of recurrence. In addition, the intra-osseous lesions in the distal tibia were not progressive during follow-up and did not cause any symptoms.
Three years after re-excision, the patient presented again with soft tissue swellings and small skin lesions producing small amounts of serous fluid. MRI was repeated and showed, as previously, multiple noduli with “dot-in-circle signs”. The patient and doctor consented to surgical re-excision to prevent further progression of the Madura foot. Again, Madurella mycetomatis was identified by fungal culture in the resected tissue. Antifungal therapy (posaconazole) was continued post-operatively.
Currently, at approximately 2.5 years after the third surgical excision and 4.5 years after the index surgical procedure, the patient is still being monitored with a 3-month interval at the infectious disease outpatient clinic and is still on posaconazole therapy (a 300 mg oral capsule once a day). Until now, there have been no signs of local recurrence. Moreover, even after three surgeries, the patient is satisfied with his current foot function, which does not limit him in his daily activities.
3.1 Methods
This review was registered at the Center for Open Science (https://www.cos.io/, last access: 7 November 2022) prior to data collection and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statements (Page et al., 2021).
3.2 Search strategy
Six literature libraries, i.e., Embase, Emcare, the Cochrane Library, Medline, PubMed, and the Web of Science, were searched for publications using a systematic search created by LH (Supplement 1). The search consisted of two components: (1) Madura foot and (2) immunocompromised patients. The list of references was exported to EndNote (version X9, Clarivate Analytics, Philadelphia, USA) to remove duplicate articles and subsequently exported to the web application Rayyan (Doha, Qatar) for study selection.
3.3 Study selection
Titles and abstracts were independently screened by two reviewers (LH and LC). Inclusion criteria were clinical studies reporting on (1) Madura foot, (2) immunocompromised patients and (3) Madurella mycetomatis as the etiological agent.
3.4 Data extraction and analysis
Data were extracted independently by LH and LC using a prespecified format (Excel version 2102, Microsoft, Redmond, USA). The following data were extracted: (a) first author, (b) year of publication, and (c) patient characteristics, including age, sex, country of origin, underlying medical condition, used immunosuppressants, initial clinical presentation, duration of complaints before first presentation, radiographical findings, histopathological assessment, treatment, duration of follow-up and outcome. The data were presented using descriptive statistics.
Literature search
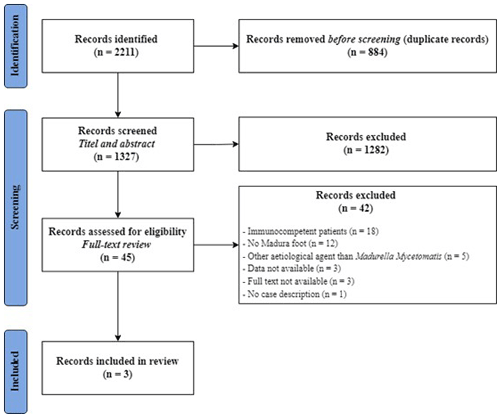
A total of 2211 publications were identified using the systematic search. After removal of duplicates, 1327 articles were screened for eligibility based on title and abstract. Thereafter, 45 full texts were assessed, of which three studies fulfilled the inclusion criteria (Fig. 5).
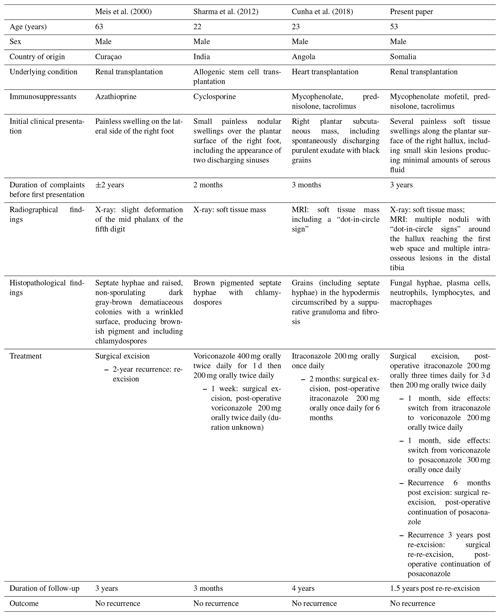
To the best of our knowledge, only three papers including a total of three immunocompromised patients presenting with a Madura foot caused by Madurella mycetomatis have been published in the literature. These cases, as well as the patient in the present paper, are summarized in Table 1. All the patients were male, with a median (range) age of 38 (22–63) years. All the patients originated from a Madura foot-endemic country, namely, Angola (Cunha et al., 2018), Curaçao (Meis et al., 2000), India (Sharma et al., 2012) and Somalia (present paper). Two patients, including ours, had received a kidney transplant (Meis et al., 2000), one a heart transplant (Cunha et al., 2018) and one an allogenic stem cell transplantation (Sharma et al., 2012). Different types of immunosuppressants were used, ranging from a broad spectrum, including mycophenolate, prednisolone and tacrolimus (Cunha et al., 2018), to azathioprine only (Meis et al., 2000).
5.1 Clinical presentation
Our patient presented with typical symptoms of a Madura foot: painless, soft tissue swellings which slowly progress over the years, with intermittent discharging granules. This is in line with the presentation of all previously reported patients, who presented with painless swellings on the plantar (Cunha et al., 2018; Sharma et al., 2012) or lateral (Meis et al., 2000) side of the foot. Furthermore, two of these three patients presented with discharging granules (Cunha et al., 2018; Sharma et al., 2012).
An untreated or insufficiently treated Madura foot can result in destruction of bone (e.g., the tibial bone) and surrounding tissues. Consequently, it may even lead to amputation (Salim et al., 2018; Gismalla et al., 2019). Fortunately, all of the described four patients were able to receive medical treatment, and amputation has (so far) not been needed in any of these patients.
5.2 Radiographic features
Although fungal culture tests are the gold standard for diagnosing a mycetoma infection (Chufal et al., 2012), radiographic analysis with plain X-rays and/or MRI are often performed to further assess the degree of bone and soft tissue involvement. A highly specific MRI finding for the diagnosis of a Madura foot, on both T1- and T2-weighted images, is the so-called “dot-in-circle sign” which was first described by Sarris et al. (2003), Cherian et al. (2009) and Parker et al. (2009). A dot-in-circle sign reflects the pathological features of the fungus and consists of a high- and low-signal area and small hypo-intense dots (Sarris et al., 2003; Cherian et al., 2009; Parker et al., 2009). The high-signal area reflects the inflammatory granulomata, whereas the low-signal area reflects the fibrous matrix and the hypo-intense foci the fungal grains (Sarris et al., 2003; Parker et al., 2009). A MRI was performed in only one of the previously reported cases (Cunha et al., 2018). It showed, similarly to our patient (Fig. 2), a “dot in circle sign” (Cunha et al., 2018). In addition, although non-specific, soft tissue masses are commonly seen on X-rays or MRI (Cunha et al., 2018; Sharma et al., 2012).
5.3 Histopathological assessment
Histopathological characteristics of Madurella mycetomatis are oval, rounded or trilobed black or dark brownish grains including septate hyphae (Chufal et al., 2012; Ibrahim et al., 2013). Histopathological assessment showed fungal hyphae in all the patients (Cunha et al., 2018; Meis et al., 2000; Sharma et al., 2012). Septate hyphae were observed in the majority of the patients (three out of four) (Cunha et al., 2018; Meis et al., 2000; Sharma et al., 2012).
5.4 Culture tests
Fungal culture tests are the gold standard for diagnosing a mycetoma infection (Chufal et al., 2012). It is important that fungal cultures are incubated for at least 4 weeks to make identification of slowly growing fungi possible; e.g., the fungal test performed in our case report became positive for Madurella mycetomatis after 22 d.
5.5 Treatment
The management of mycetoma, even when the diagnosis is made at an early stage, is challenging. Once the diagnosis is confirmed, adequate and prolonged medical therapy with triazoles is indicated (Zijlstra et al., 2016). According to current international guidelines, though not specified for immunocompromised patients, the recommended treatment is an itraconazole oral capsule of 200–400 mg once a day for a period of 6 to 9 months (Zijlstra et al., 2016). Medication alone is however rarely sufficient to cure the infection. Hence, surgical excision is often indicated for local control and to prevent progression and recurrence (Salim et al., 2018). Post-operatively, antifungal therapy has to be continued until complete clinical and radiological curation (Zijlstra et al., 2016). Unfortunately, even following long-term medical treatment and/or concomitant surgical excision, relapses of mycetoma and bone involvement of the infection often occur, which may result in subsequent surgical procedures, including amputation (Salim et al., 2018). As shown in Table 1, all four patients underwent surgical excision, two also surgical re-excision during their follow-up (range of all patients 3 months to 3 years) and three patients antifungal therapy (Cunha et al., 2018; Sharma et al., 2012). Of them, two patients received antifungal therapy (i.e., an itraconazole oral capsule of 200–400 mg once daily) according to the aforementioned guidelines (Zijlstra et al., 2016). As described earlier, the itraconazole was switched to voriconazole and eventually to posaconazole in our patient due to side effects. The patient in the case report of Meis et al. (2000) did not receive any antifungal therapy. No reason for deviation from the guidelines was described, and the reason for not prescribing antifungal therapy therefore remains unknown.
5.6 Follow-up
A Madura foot is associated with high recurrence rates (range 25 %–50 %) (Taha et al., 2015), specifically when caused by Madurella mycetomatis and/or in immunocompromised patients, despite antifungal therapy and surgical treatment. Hence, to make an early detection of a relapse achievable, it is essential to monitor patients closely, including clinical assessment of the foot, radiographical imaging and (if possible) MRI.
5.7 Immunocompromised versus immunocompetent patients
The clinical presentation of a Madura foot caused by Madurella mycetomatis is similar between immunocompromised and immunocompetent patients (Fasciana et al., 2018; Karrakchou et al., 2020; Meis et al., 2000; Asly et al., 2010; Brufman et al., 2015; Mestre et al., 2015; Sigera et al., 2020; Fida et al., 2018; Elmaataoui et al., 2011; Sampaio et al., 2015; Sharma et al., 2012; Cunha et al., 2018). However, immunocompromised patients are more likely to have a severe infection than patients who are immunocompetent, due to a weakened immune system (Knott, 2015). With regards to Madurella mycetomatis (i.e., fungi), cells of the innate immune system will be activated, aiming to eradicate the organism (Relhan et al., 2017). T-helper 2 cell (Th2) responses, producing e.g., interleukins 10 and 4, have been measured in samples of peripheral blood cells after exposure to Madurella mycetomatis antigens (Relhan et al., 2017). Besides components of the innate immune system, Th2 responses are important in the host defense to Madurella mycetomatis infection as well. Consequently, a more aggressive behavior of a Madurella mycetomatis infection may be expected in immunocompromised patients when compared to immunocompetent patients. Moreover, adequate treatment of a Madura foot caused by Madurella mycetomatis in immunocompromised patients may be more challenging when compared to the treatment of immunocompetent patients due to (1) the choice of drug regimens possibly being limited due to the risk of drug–drug interactions, (2) drug dosage adjustments possibly being required due to organ impairments involving organs responsible for drug metabolism (e.g., kidney and liver) and (3) no guidelines existing regarding the treatment and management of this disease specified for immunocompromised patients.
A Madura foot caused by Madurella mycetomatis is a rare condition in non-endemic countries. However, it should be included in the differential diagnosis in case of soft tissue swellings of the foot in patients who have been in mycetomatis-endemic regions, specifically for immunocompromised patients who are at risk of developing mycetoma and who are more likely to develop a severe infection. Adequate treatment (i.e., chronic systemic antifungal therapy, often with concomitant surgical resection) is needed to prevent progression and recurrence of the Madura foot.
The data are available in Table 1.
The supplement related to this article is available online at: https://doi.org/10.5194/jbji-7-241-2022-supplement.
LAH was responsible for the conceptualization, performed the literature search and wrote the manuscript. LSopC performed the literature search and reviewed the manuscript. AR was responsible for patient care and reviewed the manuscript. PBdW performed the surgery, was responsible for patient care and conceptualization and reviewed the manuscript. MGJdB was responsible for patient care and conceptualization and reviewed the manuscript.
The contact author has declared that none of the authors has any competing interests.
Ethical approval was not required for this study. Written informed consent was obtained from the patient.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was edited by Marjan Wouthuyzen-Bakker and reviewed by two anonymous referees.
Asly, M., Rafaoui, A., Bouyermane, H., Hakam, K., Moustamsik, B., Lmidmani, F., Rafai, M., Largab, A., and Elfatimi, A.: Mycetoma (Madura foot): A case report, Ann. Phys. Rehab. Med., 53, 650–654, https://doi.org/10.1016/j.rehab.2010.10.003, 2010.
Brufman, T., Ben-Ami, R., Mizrahi, M., Bash, E., and Paran, Y.: Mycetoma of the Foot Caused by Madurella Mycetomatis in Immigrants from Sudan, Isr. Med. Assoc. J., 17, 418–420, 2015.
Cherian, R. S., Betty, M., Manipadam, M. T., Cherian, V. M., Poonnoose, P. M., Oommen, A. T., and Cherian, R. A.: The “dot-in-circle” sign – a characteristic MRI finding in mycetoma foot: a report of three cases, Br. J. Radiol., 82, 662–665, https://doi.org/10.1259/bjr/62386689, 2009.
Chufal, S. S., Thapliyal, N. C., and Gupta, M. K.: An approach to histology-based diagnosis and treatment of Madura foot, J. Infect. Dev. Ctries., 6. 684–688, https://doi.org/10.3855/jidc.2387, 2012.
Cunha, N., Martins, M. L., Fradinho, N., Martins, P., and Cabete, J.: Plantar Eumycetoma by Madurella mycetomatis in a heart-transplanted patient living in Portugal, J. Eur. Acad. Dermatol. Venereol., 32, e182-e3, https://doi.org/10.1111/jdv.14698, 2018.
Elmaataoui, A., Elmoustachi, A., Aoufi, S., and Lyagoubi, M.: Eumycetoma due to Madurella mycetomatis from two cases of black grain mycetoma in Morocco, Journal de Mycologie Médicale, 21, 281–284, https://doi.org/10.1016/j.mycmed.2011.09.001, 2011.
El Muttardi, N., Kulendren, D., and Jemec, B.: Madura foot – mind the soil, J. Plast. Reconstr. Aesthet. Surg., 63, e576–e578, https://doi.org/10.1016/j.bjps.2009.12.007, 2010.
Fasciana, T., Colomba, C., Cervo, A., Di Carlo, P., Scarlata, F., Mascarella, C., Giammanco, A., and Cascio, A.: Madura foot: an imported case of a non-common diagnosis, Infez. Med., 26, 167–170, 2018.
Fida, M., Saraceno, R., Gjylametaj, N., Dervishi, O., Barbullushi, A., Kellici, S., and Vasili, E.: Eumycetoma pedis in an Albanian farmer, Cutis, 102, E13–e5, 2018.
Gismalla, M. D. A., Ahmed, G. M. A., MohamedAli, M. M., Taha, S. M., Mohamed, T. A., Ahmed, A. E., and Hamed, L. S.: Surgical management of eumycetoma: experience from Gezira Mycetoma Center, Sudan, Trop. Med. Health, 47, 6, https://doi.org/10.1186/s41182-018-0129-2, 2019.
Ibrahim, A. I., El Hassan, A. M., Fahal, A., and van de Sande, W. W.: A histopathological exploration of the Madurella mycetomatis grain, PLoS One, 8, e57774, https://doi.org/10.1371/journal.pone.0057774, 2013.
Karrakchou, B., Boubnane, I., Senouci, K., and Hassam, B.: Madurella mycetomatis infection of the foot: a case report of a neglected tropical disease in a non-endemic region, BMC Dermatol., 20, 1, https://doi.org/10.1186/s12895-019-0097-1, 2020.
Knott, L.: Mycetoma Maduara foot, https://patient.info/doctor/mycetoma-madura-foot (last access: 1 November 2022), 2015.
Meis, J. F., Schouten, R. A., Verweij, P. E., Dolmans, W., and Wetzels, J. F.: Atypical presentation of Madurella mycetomatis mycetoma in a renal transplant patient, Transpl. Infect. Dis., 2, 96–98, https://doi.org/10.1034/j.1399-3062.2000.020208.x, 2000.
Mestre, T., Vieira, R., and Coutinho, J.: Mycetoma of the foot–diagnosis of the etiologic agent and surgical treatment, Am. J. Trop. Med. Hyg., 93, 1–2, https://doi.org/10.4269/ajtmh.14-0651, 2015.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Schamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., McGuinness, L. A., Stewart, L. A., Thomas, J., Tricco, A. C., Welch, V. A., Whiting, P., and Moher, D.: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, Bmj, 372, 71, https://doi.org/10.1136/bmj.n71, 2021.
Parker, L., Singh, D., and Biz, C.: The dot-in-circle sign in Madura foot, J. Foot Ankle Surg., 48, 690.e1–5, https://doi.org/10.1053/j.jfas.2009.07.007, 2009.
Relhan, V., Mahajan, K., Agarwal, P., and Garg, V. K.: Mycetoma: An Update, Indian J. Dermatol., 62, 332–340, https://doi.org/10.4103/ijd.IJD_476_16, 2017.
Salim, A. O., Mwita, C. C., and Gwer, S.: Treatment of Madura foot: a systematic review, JBI Database System Rev. Implement Rep., 16, 1519–1536, https://doi.org/10.11124/JBISRIR-2017-003433, 2018.
Sampaio, F. M., Galhardo, M. C., De Farias Cardoso, R., de Oliveira Coelho, J. M. C., Lyra, M. R., and do Valle, A.C. F.: Eumycetoma on the foot caused by Madurella mycetomatis: amputation after significant worsening during pregnancy, Acta Derm. Venereol., 95, 374–375, https://doi.org/10.2340/00015555-1963, 2015.
Sarris, I., Berendt, A. R., Athanasous, N., and Ostlere, S. J.: MRI of mycetoma of the foot: two cases demonstrating the dot-in-circle sign, Skeletal Radiol., 32, 179–183, https://doi.org/10.1007/s00256-002-0600-2, 2003.
Sharma, S. K., Mukherjee, A., Singh, A. K., Seth, T., Kumar, S., Mishra, P., Xess, I., Gupta, S., Mahapatra, M., and Pati, H.: Madurella mycetomatis infection following allogenic stem cell transplantation for aplastic anemia, Mediterr. J. Hematol. Infect. Dis., 4, e2012038, https://doi.org/10.4084/MJHID.2012.038, 2012.
Sigera, L. S. M., Narangoda, K. U. L., Dahanayake, M. Y., Shabri, U. L. F., Malkanthi, M. A., Somarathne, V., Jayasekera, P. I., and Kolambage, H. A. L. P.: Mycetoma due to Madurella mycetomatis, IDCases, 21, e00857, https://doi.org/10.1016/j.idcr.2020.e00857, 2020.
Taha, H., Fahal, A., and van de Sande, W.: Mycetoma: epidemiology, treatment challenges, and progress, Research and Reports in Tropical Medicine, 6, 31–36, https://doi.org/10.2147/RRTM.S53115, 2015.
van de Sande, W. W. J.: Global burden of human mycetoma: a systematic review and meta-analysis, PLoS Negl. Trop. Dis., 7, e2550, https://doi.org/10.1371/journal.pntd.0002550, 2013.
Verma, P. and Jha, A.: Mycetoma: reviewing a neglected disease, Clin. Exp. Dermatol., 44, 123–129, https://doi.org/10.1111/ced.13642, 2019.
Welsh, O., Vera-Cabrera, L., and Salinas-Carmona, M. C.: Mycetoma, Clin. Dermatol., 25, 195–202, https://doi.org/10.1016/j.clindermatol.2006.05.011, 2007.
World Health Organization: Mycetoma 2019, https://www.who.int/news-room/fact-sheets/detail/mycetoma, last access: 14 February 2022.
Zijlstra, E. E., van de Sande, W. W. J., Welsh, O., Mahgoub, E. S., Goodfellow, M., and Fahal, A. H.: Mycetoma: a unique neglected tropical disease, Lancet Infect. Dis., 16, 100–112, https://doi.org/10.1016/S1473-3099(15)00359-X, 2016.