the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Prosthetic joint infections caused by Mycobacterium avium complex: a series of five cases
Katharine Dobos
Gina A. Suh
Aaron J. Tande
Shanthi Kappagoda
Prosthetic joint infection (PJI) due to Mycobacterium avium complex (MAC) is a rare entity. There is limited guidance on management strategies and outcomes. In this paper, we describe the demographics, comorbidities, and clinical course of five patients at two academic institutions, constituting the largest series described to date.
- Article
(555 KB) - Full-text XML
- BibTeX
- EndNote
Mycobacterium avium complex (MAC) historically represents a rare cause of prosthetic joint infection (PJI), although it remains an important pathogen to consider in routine clinical practice. Patients with immune suppression have improved survivorship and clinical stability enabling them to pursue elective orthopedic interventions, and molecular laboratory techniques have advanced to identify atypical and fastidious pathogens. Guidelines for treatment of pulmonary nontuberculous mycobacteria and PJI provide little information on how to manage PJIs caused by MAC (Daley et al., 2020; Osmon et al., 2013). Among case reports published in the literature, all of the patients reported have a comorbid immune-suppressing condition, including treated rheumatoid arthritis (RA) (Ingraham et al., 2017; Sigler and Newman, 2019), solid organ transplantation (Gupta and Clauss, 2009), hematologic malignancy (Sixt et al., 2020; Tan et al., 2016), and HIV/AIDS (McLaughlin et al., 1994). Here, we present a case series of five patients treated at two institutions with PJI caused by MAC.
After institutional review board (IRB) approval or exemption, we reviewed our electronic health records to extract information on comorbidities, diagnosis, surgical and medical therapy, and outcomes for patients with PJI caused by MAC who were treated at our respective institutions. Cases of MAC PJI between 1 January 2001 and 1 June 2021 were identified. All patients over the age of 18 years who underwent treatment for MAC PJI at our institutions were included. Medical and surgical therapies were not standardized and were performed at the discretion of the treating medical/surgical teams. The patient outcomes were followed until documentation of patient death, clinical cure, or failure to follow up at our institution. PJI was diagnosed based on provider clinical judgment at the time of intervention and was retrospectively confirmed using Musculoskeletal Infection Society (MSIS) 2011 criteria (Workgroup Convened by the MSIS, 2011). In one case, given the relative paucity of pathology and cultures available from the prosthetic joint, the diagnosis was made based on a single positive periprosthetic culture with characteristic gross operative findings combined with positive cultures from other sites in the context of disseminated infection.
The most common comorbidity among patients in our series was RA on therapy, diagnosed in three of the five patients in the series (see Table 1). Four of the five patients met MSIS 2011 (Workgroup Convened by the MSIS, 2011) criteria for PJI based on the presence of two or more positive cultures obtained from the affected prosthetic joint (see Table 2). However, we note that patient no. 5 presented with abnormal joint aspirate parameters, which may have been impacted by background hematologic derangements, and elevated inflammatory markers suspected to be confounded by active hematologic malignancy. Patient no. 4 did not clearly meet MSIS criteria but was diagnosed by means of a single positive joint aspirate culture and compelling clinical symptoms in the context of confirmed disseminated MAC.
Table 1Demographic data related to five cases of MAC PJI.
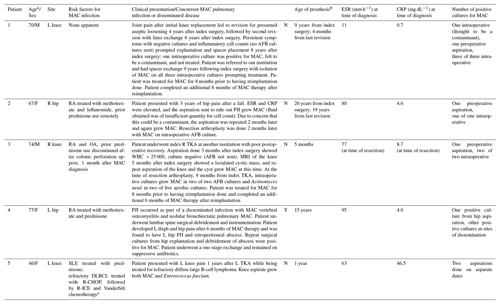
The abbreviations used in the table are as follows: AFB – acid-fast bacilli; MRI – magnetic resonance imaging; OA –
osteoarthritis; PJI – prosthetic joint infection; DLBCL – diffuse large
B-cell lymphoma; R-CHOP – rituximab,
cyclophosphamide, doxorubicin,
vincristine, and prednisone; R-ICE – rituximab, ifosfamide, carboplatin, and
etoposide; RA – rheumatoid arthritis; SLE – systemic lupus erythematosus;
TKA – total knee arthroplasty;
CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; WBC – white blood cell; M – male; F – female; L – left; R – right. a At time of diagnosis. b At time of PJI diagnosis, relative to time of index
implantation
(if not otherwise specified). c Prednisone, cyclophosphamide,
etoposide, doxorubicin, vincristine, bleomycin, methotrexate, and
leucovorin.
Among the patients in our series who underwent treatment for MAC PJI, all patients but one underwent combined medical and surgical therapy, as described in Table 2. Surgical therapy included arthroplasty resection in three patients, with two of those patients ultimately undergoing reimplantation arthroplasty. Per surgeon discretion, a spacer was not used in one patient with resection in the context of unclear benefit given preoperative diagnosis of MAC; furthermore, the patient was not anticipated to be a reimplantation candidate due to poor bone stock and surgical risk. One patient underwent debridement with modular component (femoral head and polyethylene liner) exchange. Surgery was deferred due to medical contraindications in one patient. Medical therapy included at least 12–16 months of triple-antibiotic therapy with a rifamycin, ethambutol, and macrolide in all patients, with the extension of therapy to lifelong suppression in the patient with disseminated disease. Clinical cure was achieved in three of five patients at the time of the follow-up, which ranged from 4 months to 7 years. Three of five patients treated had MAC isolates documented to be clarithromycin susceptible at the time of initiation of triple-antibiotic therapy with macrolide backbone. The clarithromycin minimum inhibitory concentration (MIC) was unknown in the remaining two.
Table 2Preoperative synovial fluid analysis and surgical findings of the current series.
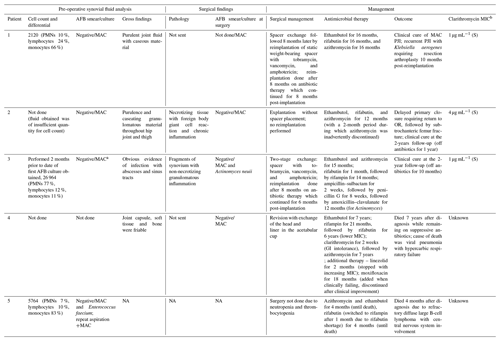
The abbreviations used in the table are as follows: PMNs – polymorphonuclear leukocytes, MIC – minimum inhibitory concentration, S – susceptible, GI – gastrointestinal, and NA – not available.
a Identified on re-aspiration 2 months following initial aspiration for cell
count/differential. b Using broth dilution method.
To date, the literature has described six cases of individuals who experienced PJI due to MAC, all of whom were considered to be immunosuppressed (Gupta and Clauss, 2009; Ingraham et al., 2017; McLaughlin et al., 1994; Sigler and Newman, 2019; Sixt et al., 2020; Tan et al., 2016). In our case series, the majority of patients (three out of five) were undergoing treatment for RA, one patient had a history of systemic lupus erythematosus (SLE) and subsequent hematologic malignancy, and one patient did not have a documented immunosuppressing risk factor for the development of MAC PJI. This raises the question of whether this patient possessed an undiagnosed immunologic deficiency which increased the risk of MAC infection, and similar cases may benefit from a formal immunology evaluation. Alternatively, there may have been a unique exposure with high inoculum load that placed this patient at risk of infection which was not captured in the medical history. Another interesting finding in this series is the absence of documented disseminated or multi-organ disease among the patients with MAC PJIs; only one of the five patients was noted to have concurrent MAC pulmonary nodular bronchiectatic (as well as native vertebral) disease. There was a wide range of prosthesis ages at the time of MAC PJI diagnosis, with a minimum of 4 months and a maximum of 26 years postoperatively, which suggests that the mechanism of inoculation may have been different among the patients described, with earlier manifestation suggesting possible introduction at time of surgery and later manifestations potentially related to hematogenous or direct traumatic seeding after an environmental exposure. In three of the five cases, MAC was the sole pathogen isolated in the orthopedic cultures. While this could raise suspicion for MAC being a contaminant rather than true pathogen, four of the patients in this series had multiple periprosthetic cultures positive for MAC (except for the patient with disseminated infection, who had a single positive culture from the affected prosthetic joint but numerous positive cultures from other sites of infection).
There was a range of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) values as well as joint fluid cell counts at the time of diagnosis, although the limited availability of a full range of data from joint aspirations preoperatively (only available for one of the five patients) makes extrapolation of the significance of this finding challenging. Of the patients who did have documented cell counts with their aspirations, only one out of the three had an elevated synovial fluid white blood cell (WBC) count and elevated PMN (polymorphonuclear leukocyte) percentage according to the 2011 MSIS criteria. Our case series shows that there can be heterogeneity in inflammatory markers and joint aspirate parameters among patients ultimately diagnosed with MAC PJI, which could be related to the severity or acuity of infection, underlying host hematologic and immunologic factors, and concurrent use of immunomodulating therapies. Outcomes among patients in our series were generally positive, with a combination of surgical management as well as prolonged (12–16 month) combination antimycobacterial therapy similar in duration to that recommended for pulmonary disease (Daley et al., 2020). However, one patient experienced another episode of PJI due to an unrelated organism after apparent cure of MAC PJI, another patient later died of an unrelated cause while remaining on suppressive MAC therapy due to disseminated disease, and yet another patient succumbed to their hematologic malignancy. This would, unsurprisingly, suggest that there is a high risk of morbidity and mortality in patients with MAC PJI due to comorbidities with MAC infection reflecting underlying host factors.
As techniques for making the diagnosis of PJI evolve, including the increasing use of molecular methods for pathogen diagnosis (which may improve the rapidity of the diagnosis of slower-growing pathogens such as MAC), we anticipate that there will be an increasing number of cases of MAC PJI described in the literature. We hope this will facilitate the development of a more cohesive strategy to identify patients at greatest risk of MAC PJI early in the disease course.
Clinicians should be aware of the potential for MAC to cause PJI in a variety of clinical contexts, and we would recommend sending AFB (acid-fast bacilli) cultures as part of the PJI workup among patients with RA or other immune-suppressing conditions as well as negative prior routine bacterial cultures.
This research is performed with IRB approval or exemption: Stanford Health Care IRB no. 45840, 03/30/2018; Mayo IRB exemption 20-010933.
Data for this project were obtained from the patients' electronic medical records. No additional data are available.
All authors were involved with preparing and editing the manuscript. SK and KD were responsible for additional contributions towards case descriptions, summary, and analysis.
At least one of the (co-)authors is a member of the editorial board of Journal of Bone and Joint Infection. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research used data or services provided by STARR (STAnford medicine Research data Repository), a clinical data warehouse containing live Epic data from Stanford Health Care, the Stanford Children's Hospital, and the University Healthcare Alliance and Packard Children's Health Alliance clinics as well as other auxiliary data from hospital applications such as radiology PACS (picture archiving and communications system). The STARR platform is developed and operated by the Stanford Medicine Research IT team and is made possible by the Stanford School of Medicine Research Office. We thank Niaz Banaei for his assistance describing microbiologic techniques and institutional protocol.
This paper was edited by Alex Soriano Viladomiu and reviewed by two anonymous referees.
Daley, C. L., Iaccarino, J. M., Lange, C., Cambau, E., Wallace, R. J., Andrejak, C., Bottger, E. C., Brozek, J., Griffith, D. E., Guglielmetti, L., Huitt, G. A., Knight, S. L., Leitman, P., Marras, T. K., Olivier, K. N., Santin, M., Stout, J. E., Tortoli, E., van Ingen, J., Wagner, D., and Winthrop, K. L.: Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline, Clin. Infect. Dis., 71, 905–913, https://doi.org/10.1093/cid/ciaa1125, 2020.
Gupta, A. and Clauss, H.: Prosthetic joint infection with Mycobacterium avium complex in a solid organ transplant recipient, Transpl. Infect. Dis., 11, 537–540, https://doi.org/10.1111/j.1399-3062.2009.00433.x, 2009.
Ingraham, N. E., Schneider, B., and Alpern, J. D.: Prosthetic Joint Infection due to Mycobacterium avium-intracellulare in a Patient with Rheumatoid Arthritis: A Case Report and Review of the Literature, Case Rep. Infect. Dis., 2017, 8682354, https://doi.org/10.1155/2017/8682354, 2017.
McLaughlin, J. R., Tierney, M., and Harris, W. H.: Mycobacterium avium intracellulare infection of hip arthroplasties in an AIDS patient, J. Bone Joint Surg. Br., 76, 498–499, 1994.
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., Rao, N., Hanssen, A., Wilson, W. R., and Infectious Diseases Society of America: Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America, Clin. Infect. Dis., 56, e1–e25, https://doi.org/10.1093/cid/cis803, 2013.
Sigler, R. and Newman, J. R.: Mycobacterium Avium Prosthetic Hip Infection on Abatacept Presenting as Fever of Unknown Origin, J. Bone Joint Infect., 4, 194–197, https://doi.org/10.7150/jbji.35703, 2019.
Sixt, T., Bador, J., Amoureux, L., Piroth, L., and Blot, M.: Prosthetic joint infection caused by Mycobacterium avium complex, Queensland Journal of Medicine, 113, 278–279, https://doi.org/10.1093/qjmed/hcz306, 2020.
Tan, E. M., Marcelin, J. R., Mason, E., and Virk, A.: Mycobacterium avium intracellulare complex causing olecranon bursitis and prosthetic joint infection in an immunocompromised host, J. Clin. Tuberc. Other Mycobact. Dis., 2, 1–4, https://doi.org/10.1016/j.jctube.2015.11.003, 2016.
Workgroup Convened by the Musculoskeletal Infection Society: New definition for periprosthetic joint infection, J. Arthroplasty, 26, 1136–1138, https://doi.org/10.1016/j.arth.2011.09.026, 2011.




