the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Defining postoperative spinal infections: navigating the inconsistencies in diagnostic definitions
Seyed Mohammad Amin Alavi
Fabio Borgonovo
Francesco Petri
Takahiro Matsuo
Andrea Gori
Jeremy D. Shaw
Aaron J. Tande
Elie F. Berbari
The absence of a standardized definition for postoperative spinal infections (PSIs) hinders both diagnosis and research. Using a meta-epidemiological approach, we analyzed 101 studies, with most relying on predefined criteria but with a minority creating their own definition (mainly clinical). Establishing a universal definition is crucial to enhancing PSI management and facilitating research.
- Article
(803 KB) - Full-text XML
-
Supplement
(965 KB) - BibTeX
- EndNote
The rising number of spine surgeries has resulted in an increased absolute burden of postoperative spinal infections (PSIs), including surgical site infections (SSIs), which are now the third most common complication following spine surgeries (Wang et al., 2022, 2023).
Previous studies have used a range of definitions for PSI, often relying on surveillance-oriented criteria that may not fully capture the clinical complexity of these infections. While the definition provided by the Centers for Disease Control and Prevention (CDC) in conjunction with the National Healthcare Safety Network (NHSN) has contributed to standardized infection surveillance, it is primarily designed for epidemiological monitoring rather than for clinical diagnosis (Tai et al., 2024). Currently, there is no universally accepted definition of PSI. The index procedure may involve instrumentation or be performed without it. Furthermore, infections can affect various anatomical sites, including the vertebrae and intervertebral discs, or may present as abscess formation. This study aimed to identify both clinical diagnostic criteria and surveillance definitions of PSI reported in the literature, using a meta-epidemiological approach. The findings can help guide expert consensus in establishing a shared definition of PSI by addressing the absence thereof, which hinders clinical practice and research by affecting population identification, and by determining desirable outcomes for clinical interventions (Kawtharany et al., 2025).
The current study followed Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines adapted for meta-epidemiological methodology (Murad and Wang, 2017). A literature search (Table S1 in the Supplement) was conducted in Ovid MEDLINE (14 March 2024) by a medical librarian, with no date restrictions but limited to English-language human studies. Studies were included if they analyzed at least 10 adults with PSI and provided a clear definition, irrespective of their purpose, either clinical or for surveillance, to ensure generalizability and comprehensiveness. The primary outcome assessed diagnostic criteria of PSIs and their thematic clustering. To standardize the landscape of definitions, 13 predefined criteria for diagnosing PSI were utilized to build combinations, based on clinical practices and prior studies (Table 1). The criteria were as follows: (1) clinical domain – systemic signs and symptoms (e.g., fever, back pain, neurological deficits); (2) clinical domain – secondary wound dehiscence; (3) clinical domain – visible/exposed implant or bone; (4) clinical domain – evidence of intraoperative or wound exudate; (5) clinical domain – inflammatory biomarkers; (6) clinical domain – benefit from conservative or operative treatment; (7) direct evidence domain – any culture from surgery; (8) direct evidence domain – culture from image-guided biopsy; (9) direct evidence domain – blood cultures; (10) direct evidence domain – culture from aspiration fluid, wound culture, or deep swab; (11) direct evidence domain – histopathology; (12) imaging – MRI; and (13) imaging – other than MRI.
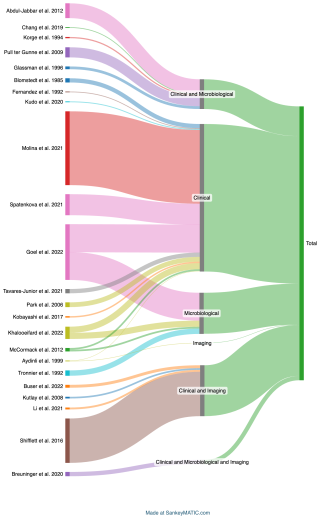
After screening 1466 articles, we included 101 that defined PSI (the PRISMA flowchart is shown in Fig. S1 in the Supplement). Most studies were retrospective (91/101, 92.1 %), with degenerative disorders being the primary indication for spine surgery (24/101, 23.8 %). The sample sizes predominantly ranged from 1000 to 10 000 total patients in each study (46/101, 45.5 %). A pre-established criterion was utilized in 77 out of 101 articles, while 7 out of 101 explicitly cited a criterion with a defined explanation in their article. The most commonly cited criterion was the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) (ACS NSQIP Participant Use Data File, 2025), cited in 53 out of 84 instances (63.1 %). Additionally, 72/101 (71.3 %) studies stratified infections as superficial or deep. Meanwhile, 24 studies provided their specific PSI definition, primarily based on clinical signs and symptoms 11/23 (47.8 %), followed by any cultures from surgery and aspiration fluid, wound culture, or deep swab (both 9/23, 39.1 %) (Table 1). Using the 13 criteria, we were not able to show any clustering of criteria.
Table 1Characteristics of the included studies.
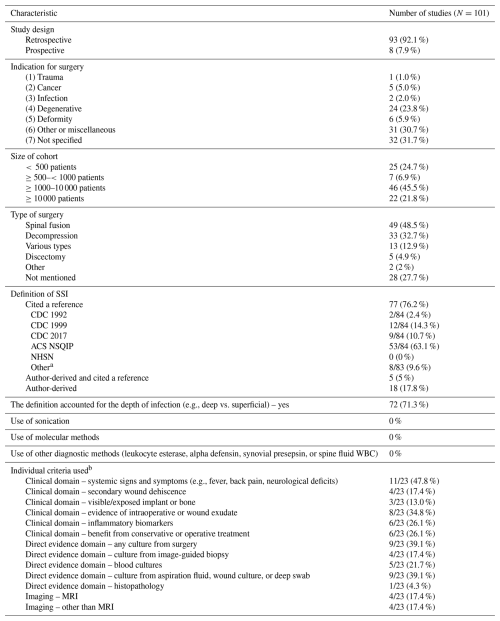
a Total can be more than 100 %, since some variables can be present in multiple categories. b This refers to 23 articles that used a definition derived by the authors. Abbreviations: American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP), Centers for Disease Control and Prevention (CDC), National Healthcare Safety Network (NHSN), Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA), magnetic resonance imaging (MRI)
Criteria were grouped as “Clinical”, “Micro” (Microbiology), and “Imaging” for better visualization. The Sankey diagram (Fig. 1) illustrates diagnostic category distribution, showing that most studies relied on clinical criteria alone, followed by clinical combined with microbiological or imaging data. Fewer cases used imaging alone or all three categories, highlighting a predominant reliance on clinical evaluation in PSI diagnosis.
Our study found that published studies used variable definitions of PSI, with the most common definitions based primarily on clinical signs and symptoms. Moreover, we showed that most previous research relied on predefined criteria for PSI, such as those established by the CDC and ACS NSQIP. While this study focuses on PSIs as a general category, we acknowledge that these infections can involve various spinal compartments, each with unique clinical characteristics and implications. Most of the included studies applied the CDC surveillance definition, classifying infections as either deep or organ/space. However, the specific anatomical sites were often not differentiated, and a broad definition of infection was commonly used.
The NHSN criteria represent one of the most recent definitions for PSI, incorporating clinical features, microbiological culture results, and imaging findings. However, similarly to earlier definitions, the NHSN criteria are primarily designed for surveillance purposes and may have limited applicability in guiding clinical decision-making (Centers for Disease Control and Prevention, 2024). Relying on this definition in clinical practice may hinder timely diagnosis and limit the identification of atypical presentations. The detailed characteristics of all criteria are summarized in Table S2. While the definitions provided by CDC, NHSN, and ACS NSQIP are largely consistent, NHSN incorporates non-culture-based diagnostic methods in the classification of superficial, deep, and organ/space SSIs. Additionally, NHSN extends the postoperative surveillance period for deep and organ/space SSIs up to 90 d, offering a more comprehensive assessment of infection risk following surgery.
The proposed methodology, designed for application across various medical fields, was tested in the transition from native vertebral osteomyelitis (NVO) (Petri et al., 2024b, a) to PSI, revealing challenges. While both conditions share similarities, PSI presents unique diagnostic complexities due to its iatrogenic etiology, variable timing from the index procedure (e.g., 6 weeks vs. 90 d), and unique clinical presentation. Diagnosis of PSI is also challenging due to the frequent presence of hardware that impacts imaging performance, the differential of wound drainage, and pseudoarthrosis or hardware failure (Tai et al., 2024). The categorization of PSI definitions must balance comprehensive yet parsimonious diagnostic criteria to avoid overfitting or underfitting. Using fewer criteria may improve clarity but risk oversimplification, while too many can create an unmanageable number of combinations. Also, the different CDC definitions for SSI updated over time are not specifically tailored for spine surgery, and the distinction between superficial, deep, and organ/space infections is not clearly categorized in the studies we reviewed. Moreover, we showed that no study specifically used sonication, molecular diagnostics, or novel biomarkers to diagnose PSI.
Similarly to what has already been done for SSI (Christensen et al., 2021; Ju et al., 2015), we highlight that, in the realm of spine surgery, NHSN and NSQIP systems often yield discordant results due to differences in case identification and definitions. Previous studies have consistently shown higher SSI rates when using ACS NSQIP, likely because the NHSN does not account for outpatient SSIs (Ju et al., 2015). Also, infection rates vary depending on the diagnostic definition applied (Nota et al., 2015). These systems rely on subjective clinical decisions, which can impact consistency in PSI rates, and we also advocate for a unified definition system.
The articles included in this study covered a broad spectrum of surgical procedures, including decompression, fusion, and discectomy, performed with or without instrumentation. Only one study specifically addressed microsurgical techniques. Efforts to standardize the definition of PSI by combining these diverse surgical approaches may affect the accuracy of infection diagnosis. Challenges to implementing a unified definition – such as variability in available resources, diagnostic limitations (e.g., imaging artifacts caused by hardware), and clinician resistance to adopting new criteria – underscore the need for future prospective studies and consensus panels. These initiatives are essential to refine the definition and classification of PSI, improve diagnostic precision, and clarify the implications for both clinical practice and infection surveillance.
This study highlights the critical need for a clear and standardized definition of postoperative spinal infection (PSI) that integrates microbiological, radiological, and clinical factors. Current surveillance systems often fail to capture the complexity of clinical presentations, leading to diagnostic uncertainty. However, the feasibility and utility of establishing a single standardized definition for all PSIs may be questionable, given the clinical heterogeneity across surgical contexts, such as the presence of hardware, procedure type, and timing of infection. While a unified framework could risk remaining a primarily academic exercise, we propose a flexible, tiered approach that accommodates these differences and supports both clinical decision-making and research. A collaborative effort is needed to develop consensus definitions that balance scientific rigor with real-world applicability.
No data sets were used in this article.
The supplement related to this article is available online at https://doi.org/10.5194/jbji-10-451-2025-supplement.
SMAA: writing (original draft and review and editing), resources, methodology, investigation, data curation, conceptualization. FB: writing (original draft and review and editing), resources, methodology, investigation, data curation, conceptualization. FP: writing (review and editing), resources, methodology, investigation, formal analysis, data curation, conceptualization. TM: writing (review and editing), methodology, investigation. AG: writing (review and editing), supervision, conceptualization. JDS: writing (review and editing), supervision, conceptualization. AJT: writing (review and editing), supervision, conceptualization. EFB: writing (review and editing), supervision, resources, project administration, methodology, investigation, conceptualization.
At least one of the (co-)authors is a member of the editorial board of Journal of Bone and Joint Infection. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Ethics approval was not required for this systematic review and meta-analysis, as the data do not provide personally identifiable information.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This paper was edited by Alex Soriano Viladomiu and reviewed by two anonymous referees.
Abdul-Jabbar, A., Takemoto, S., Weber, M. H., Hu, S. S., Mummaneni, P. V., Deviren, V., Ames, C. P., Chou, D., Weinstein. P. R., Burch, S., and Berven, S. H.: Surgical Site Infection in Spinal Surgery: Description of Surgical and Patient-Based Risk Factors for Postoperative Infection Using Administrative Claims Data, Spine, 37, https://doi.org/10.1097/BRS.0b013e318246a53a, 2012.
ACS NSQIP Participant Use Data File: https://www.facs.org/quality-programs/data-and-registries/acs-nsqip/participant-use-data-file/, last access: 23 February 2025.
Aydinli, U., Karaeminoǧullari, O., and Tişkaya, K.: Postoperative deep wound infection in instrumented spinal surgery, In Acta Orthopaedica Belgica, 65, pp. 182–187, 1999.
Blomstedt, G. C.: Infections in neurosurgery: A retrospective study of 1143 patients and 1517 operations, Acta Neurochirurgica, 78, 81–90, https://doi.org/10.1007/BF01808684, 1985.
Breuninger, M., Yagdiran, A., Willinger, A., Biehl, L. M., Otto-Lambertz, C., Kuhr, K., Seifert, H., Fätkenheuer, G., Lehmann, C., Sobottke, R., Siewe, J., and Jung, N.: Vertebral Osteomyelitis After Spine Surgery: A Disease With Distinct Characteristics, Spine, 45, https://doi.org/10.1097/BRS.0000000000003542, 2020.
Buser, Z., Chang, K.-E., Kall, R., Formanek, B., Arakelyan, A., Pak, S., Schafer, B., Liu, J. C., Wang, J. C., Hsieh, P., and Chen, T. C.: Lumbar surgical drains do not increase the risk of infections in patients undergoing spine surgery, European Spine Journal, 31, 1775–1783, https://doi.org/10.1007/s00586-022-07130-0, 2022.
Centers for Disease Control and Prevention: National Healthcare Safety Network (NHSN) Patient Safety Component Manual, Natl. Healthc. Saf. Netw. Patient Saf. Compon. Man., 1–39, https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf (last access: 23 September 2025), 2024.
Chang, C.-W., Tsai, T.-T., Niu, C.-C., Fu, T.-S., Lai, P.-L., Chen, L.-H., and Chen, W.-J.: Transforaminal Interbody Debridement and Fusion to Manage Postdiscectomy Discitis in Lumbar Spine, World Neurosurgery, 121, 755–760, https://doi.org/10.1016/j.wneu.2018.09.211, 2019.
Christensen, A. M. M., Dowler, K., and Doron, S.: Surgical site infection metrics: Dissecting the differences between the National Health and Safety Network and the National Surgical Quality Improvement Program, Antimicrob. Steward. Healthc. Epidemiol., 1, e16, https://doi.org/10.1017/ash.2021.176, 2021.
Fernandez, M. C., Gottlieb, M., and Menitove, J. E.: Blood transfusion and postoperative infection in orthopedic patients, Transfusion, 32, 318–322, https://doi.org/10.1046/j.1537-2995.1992.32492263444.x, 1992.
Glassman, S. D., Dimar, J. R., Puno, R. M., and Johnson, J. R.: Salvage of instrumental lumbar fusions complicated by surgical wound infection, Spine, 21, 2163–2169, https://doi.org/10.1097/00007632-199609150-00021, 1996.
Goel, V., Kaizer, A., Patwardhan, A. M., Ibrahim, M., DeSimone, D. C., Sivanesan, E., and Shankar, H.: Postoperative Oral Antibiotic Use and Infection-Related Complications After Spinal Cord Stimulator Surgery, Neuromodulation, 25, 738–744, https://doi.org/10.1016/j.neurom.2021.10.012, 2022.
Ju, M. H., Ko, C. Y., Hall, B. L., Bosk, C. L., Bilimoria, K. Y., and Wick, E. C.: A Comparison of 2 Surgical Site Infection Monitoring Systems, JAMA Surg., 150, 51–57, https://doi.org/10.1001/jamasurg.2014.2891, 2015.
Kawtharany, H., Azzam, M., Murad, M. H., Morgan, R. L., Falck-Ytter, Y., Sultan, S., Dahm, P., and Mustafa, R. A.: Proposed framework for unifying disease definitions in guideline development, BMJ Evidence-Based Med., https://doi.org/10.1136/bmjebm-2024-113134, 2025.
Khalooeifard, R., Oraee-Yazdani, S., and Vahdat Shariatpanahi, Z.: Obesity and posterior spine fusion surgery: A prospective observational study, International Journal of Orthopaedic and Trauma Nursing, 45, 100920, https://doi.org/10.1016/j.ijotn.2021.100920, 2022.
Kobayashi, K., Imagama, S., Ito, Z., Ando, K., Yagi, H., Hida, T., Ito, K., Ishikawa, Y., Tsushima, M., and Ishiguro, N.: Is a Drain Tip Culture Required After Spinal Surgery? Clinical Spine Surgery, 30, https://doi.org/10.1097/BSD.0000000000000326, 2017.
Korge, A., Fischer, R., Kluger, P., and Puhl, W.: The importance of sonography in the diagnosis of septic complications following spinal surgery, European Spine Journal, 3, 303–307, https://doi.org/10.1007/BF02200141, 1994.
Kudo, Y., Okano, I., Toyone, T., Matsuoka, A., Maruyama, H., Yamamura, R., Ishikawa, K., Hayakawa, C., Tani, S., Sekimizu, M., Hoshino, Y., Ozawa, T., Shirahata, T., Fujita, M., Oshita, Y., Emori, H., Omata, H., and Inagaki, K.: Lateral lumbar interbody fusion in revision surgery for restenosis after posterior decompression, Neurosurgical Focus FOC, 49, 11, https://doi.org/10.3171/2020.6.FOCUS20361, 2020.
Kutlay, M., Colak, A., Simsek, H., Yildiz, S., Topuz, K., Kaya, S., Cetinkal, A., and Demircan, M.: Antibiotic and hyperbaric oxygen therapy in the management of post-operative discitis, Undersea & Hyperbaric Medicine: Journal of the Undersea and Hyperbaric Medical Society, Inc, 35, 427–440, 2008.
Li, Y.-D., Chi, J.-E., Chiu, P.-Y., Kao, F.-C., Lai, P.-L., and Tsai, T.-T.: The comparison between anterior and posterior approaches for removal of infected lumbar interbody cages and a proposal regarding the use of endoscope-assisted technique, Journal of Orthopaedic Surgery and Research, 16, 386, https://doi.org/10.1186/s13018-021-02535-x, 2021.
McCormack, R. A., Hunter, T., Ramos, N., Michels, R., Hutzler, L., and Bosco, J. A.: An Analysis of Causes of Readmission After Spine Surgery, Spine, 37, https://doi.org/10.1097/BRS.0b013e318245f561, 2012.
Molina, E., Zhao, D., Dowlati, E., Carroll, A. H., Mueller, K. B., Sandhu, F. A., and Voyadzis, J.-M.: Minimally invasive posterior lumbar surgery in the morbidly obese, obese and non-obese populations: A single institution retrospective review, Clinical Neurology and Neurosurgery, 207, 106746, https://doi.org/10.1016/j.clineuro.2021.106746, 2021.
Murad, M. H. and Wang, Z.: Guidelines for reporting meta-epidemiological methodology research, BMJ Evidence-Based Med., 22, 139–142, https://doi.org/10.1136/ebmed-2017-110713, 2017.
Nota, S. P. F. T., Braun, Y., Ring, D., and Schwab, J. H.: Incidence of surgical site infection after spine surgery: what is the impact of the definition of infection?, Clin. Orthop. Relat. Res., 473, 1612–1619, https://doi.org/10.1007/s11999-014-3933-y, 2015.
Park, M.-S., Moon, S.-H., Kim, H.-S., Hahn, S.-B., Park, H.-W., Park, S.-Y., and Lee, H.-M.: A Comparison of Autologous and Homologous Transfusions in Spinal Fusion, Yonsei Med J, 47, 840–846, https://doi.org/10.3349/ymj.2006.47.6.840, 2006.
Petri, F., Mahmoud, O., El Zein, S., Nassr, A., Freedman, B. A., Verdoorn, J. T., Tande, A. J., and Berbari, E. F.: It is time for a unified definition of native vertebral osteomyelitis: a framework proposal, J. Bone Joint Infect., 9, 173–182, https://doi.org/10.5194/jbji-9-173-2024, 2024a.
Petri, F., Mahmoud, O. K., Zein, S. El, Alavi, S. M. A., Passerini, M., Diehn, F. E., Verdoorn, J. T., Tande, A. J., Nassr, A., Freedman, B. A., Murad, M. H., and Berbari, E. F.: Wide Variability of the Definitions Used for Native Vertebral Osteomyelitis: Walking the Path for a Unified Diagnostic Framework with a Meta-Epidemiological Approach, Spine J., https://doi.org/10.1016/j.spinee.2024.09.018, 2024b.
Pull ter Gunne, A. F. and Cohen, D. B.: Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery, Spine, 34, 1422–1428, https://doi.org/10.1097/BRS.0b013e3181a03013, 2009.
Shifflett, G. D., Bjerke-Kroll, B. T., Nwachukwu, B. U., Kueper, J., Burket, J., Sama, A. A., Girardi, F. P., Cammisa, F. P., and Hughes, A. P.: Microbiologic profile of infections in presumed aseptic revision spine surgery, European Spine Journal, 25, 3902–3907, https://doi.org/10.1007/s00586-016-4539-8, 2016.
Spatenkova, V., Bradac, O., Jindrisek, Z., Hradil, J., Fackova, D., and Halacova, M.: Risk factors associated with surgical site infections after thoracic or lumbar surgery: a 6-year single centre prospective cohort study, Journal of Orthopaedic Surgery and Research, 16, 265, https://doi.org/10.1186/s13018-021-02418-1, 2021.
Tai, D. B. G., Patel, R., Lovecchio, F., Kwee, T., and Wouthuyzen-Bakker, M.: Executive Summary: State-of-the-Art Review: Diagnosis and Management of Spinal Implant Infections., Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am., 79, 1329–1330, https://doi.org/10.1093/cid/ciae432, 2024.
Tavares-Júnior, M. C. M., Cabrera, G. E. D., Teixeira, W. G. J., Narazaki, D. K., Ghilardi, C. S., Marcon, R. M., Cristante, A. F., and de Barros-Filho, T. E. P.: Risk Factors Associated with Postoperative Infection in Cancer Patients Undergoing Spine Surgery, Clinics, 76, 2741, https://doi.org/10.6061/clinics/2021/e2741, 2021.
Tronnier, V., Schneider, R., Kunz, U., Albert, F., and Oldenkott, P.: Postoperative spondylodiscitis: Results of a prospective study about the aetiology of spondylodiscitis after operation for lumbar disc herniation, Acta Neurochirurgica, 117, 149–152, https://doi.org/10.1007/BF01400612, 1992.
Wang, S., Wang, P., Li, X., Sun, W., Kong, C., and Lu, S.: Enhanced recovery after surgery pathway: association with lower incidence of wound complications and severe hypoalbuminemia in patients undergoing posterior lumbar fusion surgery, J. Orthop. Surg. Res., 17, 178, https://doi.org/10.1186/s13018-022-03070-z, 2022.
Wang, X., Lin, Y., Yao, W., Zhang, A., Gao, L., and Feng, F.: Surgical site infection in spinal surgery: a bibliometric analysis, J. Orthop. Surg. Res., 18, 337, https://doi.org/10.1186/s13018-023-03813-6, 2023.