the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Proactive Therapeutic Drug MONiToring to Guide Suppressive Antibiotic Therapy with DALBAvaNcin ( > 12 weeks) in Osteoarticular Infections (MONTALBANO)
Chiara Mariani
Matteo Passerini
Lucia Galli
Alice Covizzi
Marta Colaneri
Martina Offer
Margherita Faenzi
Stefania Merli
Simona Landonio
Marta Fusi
Alberto Dolci
Andrea Gori
Dario Cattaneo
Introduction: Long-term dalbavancin use is increasingly adopted off-label for osteoarticular infections (OAIs), but data on administration timing and long-term effects beyond 12 weeks are scarce. This study evaluated the pharmacological efficacy of proactive therapeutic drug monitoring (TDM) to optimize dalbavancin administration. Methods: This single-center, retrospective study included adult OAI patients treated with ≥4 doses of dalbavancin from July 2022 to October 2024. Initial doses were given on days 1, 8, and 43. From the third dose onward, Cmin and Cmax values informed dosing schedules via log-linear regression models, targeting mg L−1. The primary outcome was the pharmacological efficacy of dalbavancin, assessed by the proportion of patients with mg L−1 and ≥4 mg L−1 after the third dose. Clinical outcomes and safety data were collected as descriptive data. Results: A total of 33 patients provided 118 Cmin determinations. Pharmacological efficacy was achieved in 93118 (78.8 %) and 114118 (96.6 %) determinations for Cmin thresholds of ≥8 mg L−1 and ≥4 mg L−1, respectively. Efficacy improved when considering only determinations at the correct timing. A total of 18 (54.5 %) patients are still in treatment, while 11 (33.3 %) completed therapy with clinical success. Three patients experienced a relapse after the end of the treatment, while one patient experienced failure, and no adverse events were reported. Conclusions: Dalbavancin is a viable option for prolonged OAI management when other therapies are unavailable or high-risk. Proactive TDM effectively supports this approach by ensuring adequate drug exposure while preventing accumulation.
- Article
(1182 KB) - Full-text XML
- BibTeX
- EndNote
Dalbavancin is a long-acting lipoglycopeptide derived from a teicoplanin-like antibiotic (Malabarba and Goldstein, 2005) with activity against gram-positive bacteria (Weber et al., 2021) (Mendes et al., 2016).
Dalbavancin is characterized by a long half-life (14.4 d) and a total binding protein of 93 % (Rappo et al., 2019a). It is primarily cleared through renal pathways, lacks active metabolites, and is unaffected by cytochrome P450 inhibitors or other enzyme-inducing drugs (Buckwalter and Dowell, 2005). It was approved by the Food and Drug administration (FDA) in 2014 and subsequently by the European Medicines Agency (EMA) in 2015 for the treatment of acute bacterial skin and skin structure infections (ABSSIs) in one dose of 1500 mg or in two doses of 1000 and 500 mg 1 week apart (Boucher et al., 2014; Dunne et al., 2016). The drug is generally well tolerated, with nausea, headache, diarrhoea, vomiting, and mild rash occurring as the most common adverse events (AEs) (Gatti et al., 2021), while severe AEs such as acute renal injury or anaphylaxis reactions occurred only rarely (Bork et al., 2019).
Dalbavancin is also characterized by a decent penetration proportion (13.1 %) into bone tissue (Dunne et al., 2015) and into staphylococcal biofilms (Silva et al., 2021), making this antibiotic a promising weapon for the treatment of osteoarticular and biofilm-mediated infections. Thus, dalbavancin showed increasing evidence of efficacy in off-label regimens for infections due to gram-positive multidrug-resistant (MDR) organisms requiring prolonged treatment such as osteoarticular infections (OAIs) (Rappo et al., 2019b; Esposito et al., 2024). Moreover, dalbavancin, with its extended dosing interval, represents a valuable alternative to daily IV antibiotics, particularly in cases where oral therapy is either unfeasible or contraindicated (Azamgarhi et al., 2025). Dalbavancin may also be suitable for clinical scenarios requiring suppressive antibiotic therapy (SAT), a practice that still lacks clear guidelines for optimal management (Horne et al., 2024).
Despite its increasing use, the timing and dosage for off-label and long-term administration of dalbavancin remain undefined. Recent evidence supports the use of therapeutic drug monitoring (TDM) to estimate the duration of effective treatment in patients with OAIs (Cojutti et al., 2021). In addition, a recent expert opinion proposed regimens to achieve adequate dalbavancin concentration of up to 12 weeks (Senneville et al., 2023). Nonetheless, data on treatment durations beyond this period are still limited, and no specific recommendations are currently available regarding administration schedules or potential long-term effects.
At our center, as observed in other settings (Wouthuyzen-Bakker et al., 2017; Hanssen et al., 2024), we identified a subgroup of patients with OAIs, particularly hardware-associated infections, who require treatment exceeding 12 weeks due to a high risk of failure with shorter regimens and ineligibility for revision surgery. To address this, we developed a TDM-based approach to proactively guide dalbavancin administration in treatments lasting longer than 12 weeks (Cattaneo et al., 2024). Thus, the aim of this study is to assess the pharmacological efficacy of our method for OAIs and, secondly, to evaluate its clinical effectiveness and safety. Building on our previous data (Cattaneo et al., 2023, 2024), this study focuses exclusively on TDM observations relevant to extending treatment beyond 12 weeks, specifically for OAIs. It also expands the patient cohort, including clinical data on baseline characteristics, efficacy, and safety.
2.1 Study design and setting
We conducted a single-center observational study from the registry of off-label drugs considering patients with OAIs treated with dalbavancin at Luigi Sacco Hospital, in the Outpatients Parenteral Antimicrobial Therapy (OPAT) clinic, from July 2022 to October 2024. After planning the study and the research questions, we retrospectively collected data.
2.2 Participants
We included all adult patients (>18 years) who received at least four administrations of dalbavancin for OAIs and for whom TDM was performed starting from at least the fourth dose onward. We included only cases with at least four administrations of dalbavancin because the first three doses are administered at predefined time points, whereas the fourth dose is the first one adjusted based on the pharmacological method applied at our center (see Sect. 2.3). We excluded pediatric patients, patients receiving fewer than four dalbavancin administrations, patients without information about estimated data for the fourth dalbavancin administration, patients without minimum plasma concentrations (Cmin) at the fourth dose, patients with indications other than OAIs, and patients without informed consent.
2.3 Timing of dalbavancin administrations
Included patients received the first three dalbavancin doses on day 1 (T0), day 8 (T1), and day 43 (T2) (Senneville et al., 2023). Subsequent injection timings were personalized and determined using dalbavancin Cmin measured immediately before the dalbavancin administration and maximum plasma concentrations (Cmax) measured 30 min after the end of the injection (Fig. 1). Starting from the third dose, Cmin and Cmax values were used in log-linear regression models by plotting logarithmic dalbavancin concentrations with time (Microsoft Excel Software, Microsoft Company, USA) (Cattaneo et al., 2023) to plan the subsequent administration aiming to maintain Cmin levels at ≥8 mg L−1, as recommended in the recent literature (Cojutti et al., 2021).
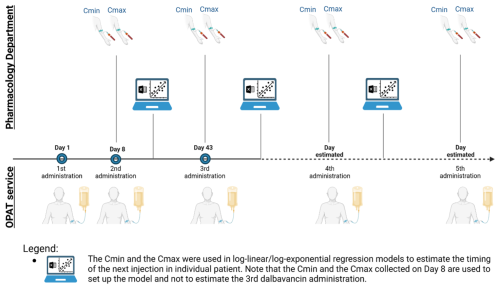
Figure 1Clinical practice in OPAT service applying a TDM-based method to plan dalbavancin administrations for a duration of treatment longer than 12 weeks. Created in https://BioRender.com (last access: 5 May 2025).
2.4 Assessment of plasma dalbavancin concentrations
Plasma dalbavancin blood samples were assessed using ethylene–diamine–tetraacetic acid (EDTA)-containing Vacutainers®. All samples were centrifuged at 3000 × g and plasma was separated and stored at −20 °C. Dalbavancin quantification was performed through a validated liquid chromatography tandem mass spectrometry method developed and validated according to the EMA guidelines. The analytical process consisted of a fast protein precipitation protocol of 50 µL of plasma with 400 µL of precipitation solution (methanol acetonitrile, 3 1), centrifugation at 10 000 × g, 1 : 3 dilutions with water, and analysis. Chromatographic separation was achieved using a gradient (acetonitrile and water with formic acid 0.1 %) on a reversed-phase analytical column (Acquity UPLC BEH C18 1.70 µm 2.1 × 50 mm; Waters, Milan, Italy). For quantification, analysis was performed in ESI-positive mode by monitoring the transition = 909.45 > 340.2 for dalbavancin. The method was linear from 1 to 500 mg L−1, with intraday and interday assay imprecision and inaccuracy consistently < 10 % during each analytical run.
2.5 Primary outcome
The primary outcome was to evaluate the pharmacological efficacy of our proactive TDM-based administration method in maintaining the dalbavancin Cmin level at ≥8 mg L−1 or ≥4 mg L−1 taken from the fourth dose onward.
2.6 Other descriptive data of interest
We also collected clinical effectiveness and safety data. Given the observational nature of the study and the limited sample size, these outcomes were collected as descriptive data.
2.7 Definitions
2.7.1 Pharmacological efficacy
Pharmacological efficacy was defined as the number of instances where mg L−1(or ≥4 mg L−1) was achieved from the fourth dose onward. The thresholds of 4 and 8 mg L−1 were selected based on prior literature, which suggests that these concentrations ensure, with approximately 90 % probability, the attainment of pharmacodynamic targets against Staphylococcus aureus for MIC values up to MIC90 (0.0625 mg L−1) and the EUCAST clinical susceptibility break point for dalbavancin (0.125 mg L−1), respectively (Dunne et al., 2015; Cojutti et al., 2021). A tolerance window of up to ±4 d from the calculated injection date was allowed to adapt the model to real-life conditions and improve adherence to the treatment regimen.
2.7.2 Clinical effectiveness
We established effectiveness with an assessment of clinical and microbiological outcomes from chart review detected up to 31 October 2024. If the patient was still on dalbavancin treatment, the clinical outcome was assessed as “ongoing treatment”. Otherwise, the assessment could result in the following: (i) completed therapy with clinical success (absence of failure or relapse at the follow-up); (ii) failure (clinical or microbiological failure with uncontrolled infection during dalbavancin treatment; need for medical interventions for the treated episode in addition to the initial program); (iii) relapse (exacerbation of the clinical condition within 6 months after the end of treatment); (iv) discontinuation of therapy due to AEs; (v) discontinuation of therapy due to new hospitalization for other clinical events; (vi) missing data (insufficient details to assess the outcome; lost at follow-up); (vii) death for infection-related conditions; or (viii) death for non-infection-related conditions.
2.7.3 Safety
Safety was assessed by monitoring AEs. We defined renal toxicity as a reduction in the estimated glomerular filtration rate (eGFR) to less than 30 mL min−1/1.73 m2, accompanied by a decrease of at least 20 % from baseline. A significant reduction in platelet count was considered when the count fell below 100 000 per mm−3 with a decrease of at least 25 % from baseline.
2.8 Data source and extraction
We identified the patients from the registry of off-label drugs and the registry of the OPAT clinic, which collects all the individuals receiving dalbavancin in an off-label regimen. Then we collected data from electronic records using “NOEMALIFE Galileo” software and manual chart review from the OPAT clinic. Extracted data included age, sex, body mass index (BMI), comorbidities, type of OAI, presence of hardware, isolated pathogens, concomitant antibiotics, source control, number of doses and dosage of dalbavancin, duration of dalbavancin therapy, information about the fourth dalbavancin administration and the subsequent administrations (date estimated by log-linear regression method for antibiotic administration, date effective of antibiotic administration, dalbavancin Cmin concentration), laboratory tests (serum albumin at T0; platelet count, serum creatinine, and eGFR at baseline (T0) and at 7±3 d of therapy (T1), at 6 weeks ± 10 d of therapy (T2), at 12 weeks ± 10 d of therapy (T3), at 6 months of therapy (T4), at 12 months of therapy (T5) and 18 months of therapy (T6)), potentially related AEs (significant platelet count reduction, acute renal failure, dermatological manifestation, angioedema, and other), clinical outcome, and duration of follow-up. Data were recorded in Microsoft Excel v16.0.
2.9 Statistical methods
To determine the timing of dalbavancin administration, refer to Sect. 2.3. To describe the baseline characteristics and the outcomes, we described continuous variables as a median (interquartile range – IQR), while categorical variables are described as counts and percentages of positive responses.
3.1 Participants
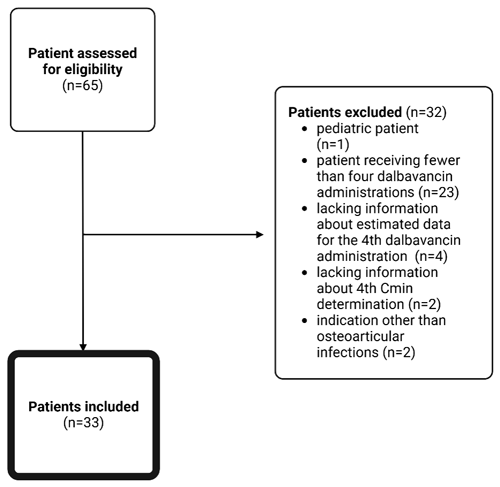
The registries of the off-label drugs and OPAT clinic identified 65 patients. The final analysis included 33 patients (Fig. 2).
3.2 Descriptive data
Most of the included patients were males (2033, 60.6 %); median age was 73 years (interquartile range, IQR: 58–78) and median BMI was 25.7 (IQR, 23.7–27.7), identifying a median overweight population. A total of 25 out of 33 patients (75.8 %) had hardware-associated bone and joint infections. Median (range, minimum to maximum) baseline serum albumin, creatinine, and eGFR were 36 (25 to 47) g L−1, 0.97 (0.45 to 2.07) mg dL−1, and 81.25 (17.2 to 214.1) mL min−1/1.73 m2, respectively. Patients received a median of 5 (range, 4–18) injections over a median period of 133 d (IQR, 87–226) between the first and the last dalbavancin administration. All patients received 1500 mg of dalbavancin as a first dose; 3133 (93.9 %) continue with this dosage, while 233 (6.1 %) patients received 1000 mg in a maintenance dose due to their chronic kidney disease (baseline eGFR was 35.2 and 17.2 mL min−1/1.73 m2, respectively). Additional patient demographic and clinical characteristics are summarized in Table 1. We observed substantial variability in administration intervals across patients, whereas intra-patient variability remained relatively limited and was further reduced upon exclusion of administrations occurring more than 7 d beyond the recommended time frame (Fig. 3).
Table 1Baseline characteristics of the study population.
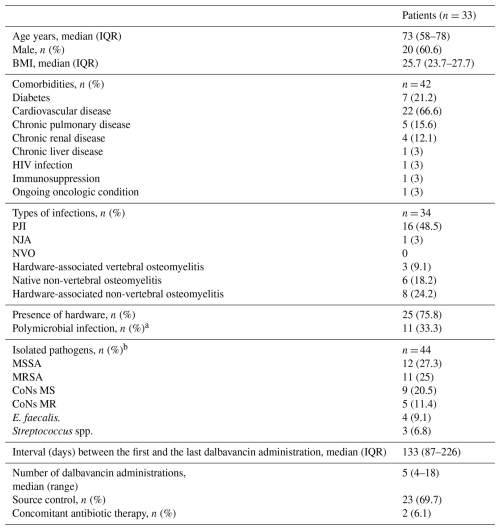
The abbreviations used in the table are as follows. IQR: interquartile range; BMI: body mass index; HIV: human immunodeficiency virus; NJA: native joint arthritis; NVO: native vertebral osteomyelitis; MSSA: methicillin-sensitive Staphylococcus aureus; MRSA: methicillin-resistant Staphylococcus aureus; CoNS MS: methicillin-sensitive coagulase-negative staphylococcus; CoNS MR: methicillin-resistant coagulase-negative staphylococcus. a 1011 due to gram-positive pathogens, 111 due to gram-negative pathogens. b MIC for vancomycin for 19 isolates: 219 MIC < 1, 1319 MIC = 1, 219 MIC = 2; we calculated MIC for dalbavancin just for one patient and it resulted in < 0.06.
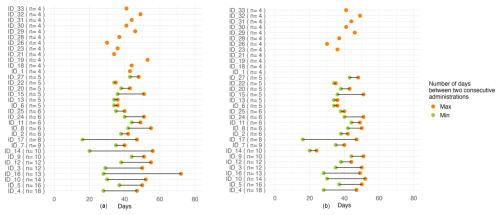
Figure 3The left panel represents the interval between dalbavancin administrations in days (after excluding the first three doses). The number in parentheses indicates the number of dalbavancin administrations received by each patient. The right panel represents the same interval after excluding the administrations beyond 7 d from the suggested date (note the difference in patients ID_14 and ID_16).
3.3 Primary outcome
A total of 93 of 118 (78.8 %) and 114118 (96.6 %) Cmin resulted in ≥8 mg L−1 and ≥4 mg L−1, respectively. A total of 95 of 118 (80.5 %) through concentrations (Cmin) were performed in the time window suggested by the model; among these 8195 (85.3 %) were ≥8 mg L−1 and 9395 (97.9 %) were ≥4 mg L−1 (Table 2).
3.4 Secondary outcomes
A total of 18 out of 33 (54.5 %) patients were still undergoing treatment with controlled infection at the end of the study, and 1133 (33.3 %) completed therapy with clinical success. Three patients experienced a relapse of osteomyelitis: one at the level of the fifth metatarsal 6 months after completing therapy and two others approximately 2 months post-therapy, involving the ankle and leg, respectively, in regions where hardware remained in situ. Finally, one patient had to discontinue dalbavancin due to a new hospitalization for a new infection, possibly related to the original focus; we considered it a failure. All four patients with relapse or failure had at least one mg L−1, and had at least one value <4 mg L−1. Among the 29 patients with clinical success, 15 had at least one mg L−1 and 12 had at least one <4 mg L−1.
None of the 33 included patients experienced AEs (Table 3).
Our study collected data from a cohort of patients with OAIs treated with dalbavancin for over 12 weeks, utilizing a proactive TDM-based approach to evaluate its pharmacological efficacy, clinical effectiveness, and safety. Pharmacological efficacy was achieved in more than three-quarters of the study population based on the concentration targets reported in the literature for OAIs caused by Staphylococcus aureus. Furthermore, most of the study population either continued treatment or achieved clinical success without AEs, suggesting the clinical effectiveness and safety of our TDM-based approach.
Currently, there is considerable heterogeneity in the use of SAT with dalbavancin for OAIs in both the initial and the maintenance doses. For the latter, some proposed regimens range from 500 mg weekly to 500 mg biweekly to 1000 mg biweekly (Martín et al., 2019; Morata et al., 2019; Wunsch et al., 2019). However, none of these studies employed TDM to assess the pharmacological efficacy of such regimens. Söderquist et al. (2024) recently shared their experience with 12 patients treated with long-term (>12 weeks) dalbavancin for PJI, according to the Swedish national guidelines and introducing TDM. These patients received 1500 mg on day 1, day 8, and day 14, followed by 1000 mg biweekly starting on day 28. Prospective sampling for dalbavancin Cmin was conducted biweekly. All resulting Cmin values were well above the therapeutic target of 8 mg L−1, and three patients showed a tendency towards progressive accumulation of dalbavancin during treatment (Söderquist et al., 2024).
In this context, our study introduces a novel TDM-based approach to manage long-term dalbavancin treatment for OAIs requiring more than 12 weeks of therapy. Most of the Cmin values obtained with our method remained above the therapeutic target without leading to drug accumulation. Notably, our results improved when we considered only Cmin measurements collected within in the time window recommended by our method. Our approach, in most cases, ensures adequate drug levels and prevents sub-therapeutic serum concentrations, thereby potentially reducing the risk of failure and development of antibiotic resistance. Secondly, this proactive approach enables the prevention of dalbavancin accumulation rather than requiring dose adjustments after accumulation has already occurred. Specifically, we did not observe any adverse effects on renal function or platelet count, which could have been expected given the known potential adverse effects of glycopeptide-derived drugs.
Our TDM approach is hypothesized to have a positive impact not only on efficacy and safety but also on resource management. Administering the correct dose to patients while avoiding unnecessary additional doses can lead to significant cost savings, particularly given the relatively low cost of a single TDM assay or the use of a clinical pharmacology reference center serving as a hub for multiple facilities. Furthermore, since dalbavancin's Cmin and Cmax measurements, as well as its administration, are conducted within the same half-day, this strategy also saves time and resources for both patients and healthcare staff. TDM was performed when the patient came for the next dose, which suits OPAT logistics. However, based on our observations, intra-patient variability was higher in the first four to five doses and tended to stabilize later. Thus, TDM might be more critical early in treatment and less frequent thereafter.
Our study has several limitations. First, its retrospective design results in significant missing data, such as minimum inhibitory concentration (MIC) values for dalbavancin and variability in follow-up duration, which could potentially affect the accuracy of clinical outcome data related to effectiveness. Second, we included a heterogeneous and small population, and we did not use a comparator group without TDM-based approach. This does not allow us to assess effectiveness and safety with strong evidence. However, the primary outcome of our study was to assess the pharmacological efficacy of our approach. Third, the choice of 8 and 4 mg L−1 as Cmin targets is based on studies conducted on Staphylococcus aureus, rather than on our study population, which also included OAIs caused by other pathogens, such as Enterococcus faecalis and Streptococcus spp. The 8 mg L−1 target derives from literature on protein binding, bone penetration, and EUCAST MICs for S. aureus. However, the optimal target likely varies with the pathogen's MIC and infection site. We also suspect serum albumin may affect the active drug fraction, but further data are needed. Fourth, there were unexpected events related to clinical practice. Specifically, from 15 February to 24 April 2024, our Pharmacology Department was unable to perform dalbavancin TDM due to machinery failure, and therefore the appointments were based on previous calculated treatment intervals. Additionally, in May 2024, insufficient drug availability led to one patient receiving oral therapy with trimethoprim–sulfamethoxazole for 1 month before resuming dalbavancin. During the same period, one patient previously considered for suppressive antibiotic therapy interrupted treatment from 4 to 30 May 2024 before restarting dalbavancin infusions. Lastly, we measured total concentration rather than free concentration, which is thought to be more reliable, since free concentration–time profiles may best correlate with therapeutic effect. Nevertheless, measuring free concentrations is technically challenging, resource-intensive, and not yet feasible for routine clinical practice.
We also acknowledge two factors that currently limit the external applicability of this method. The first is that not all centers are equipped with a clinical pharmacology service capable of performing TDM and supporting patient care. The second is that prolonged use of dalbavancin may be economically unsustainable for some centers. The first limitation can be mitigated, as previously mentioned, by establishing a center that acts as a regional hub for other facilities. Additionally, one of the future goals of our working group is to develop a model that can be utilized even when TDM values are not available. Although preliminary analyses suggested that variables such as BMI may influence dosing intervals, the limited dataset in this study rendered the statistical analyses unreliable. We plan to expand the cohort to better investigate these correlations and potentially incorporate them into the proposed model. The second limitation necessitates careful patient selection to identify those who are most likely to benefit from dalbavancin use. For instance, as has been recently emphasized, every effort should be made to switch from IV to oral therapy whenever feasible and when the expected benefits outweigh the risks, considering the individual patient's circumstances and antimicrobial stewardship practices (Senneville et al., 2023). Furthermore, we believe that collaboration with national regulatory authorities is essential to facilitate easier access to the drug, even for off-label use, in cases where other therapeutic options are unavailable.
Despite these limitations, our study highlights the potential role of dalbavancin in long-term treatment. This role could be further enhanced by a TDM-based approach, which enables more precise timing of dalbavancin administration. Such guidance ensures adequate drug concentrations, minimizes the risks of accumulation and associated adverse events, prevents sub-therapeutic serum levels that could reduce effectiveness, and avoids unnecessary economic waste.
The code and data used in this work are available from the corresponding author upon request.
CM: conceptualization, data curation, investigation, writing (original draft) MP: conceptualization, investigation, methodology, validation, writing (original draft), visualization. LG: data curation, investigation. AC: resources, methodology, investigation. MC: conceptualization, methodology, writing (review and editing). MO: methodology, software, formal analysis. MF: resources, writing (review and editing). SM: conceptualization, data curation, writing (review and editing). SL: conceptualization, data curation, writing (review and editing). MF: software, formal analysis, writing (review and editing). AD: resources, writing (review and editing). AG: supervision, funding acquisition, writing (review and editing). DC: conceptualization, investigation, methodology, validation, formal analysis, writing (review and editing). All authors have read and agreed to the published version of the paper.
Andrea Gori received a fee from Janssen, ViiV, MSD, BMS, Abbvie, Gilead, Novartis, Pfizer, Astellas, Astrazeneca, and Angelini. Dario Cattaneo received a speaker's fee from Pfizer, MSD, and Gilead and educational grants from ViiV Healthcare. However, all the authors declare that they do not have known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The study was conducted in accordance with the dictates of the Declaration of Helsinki and the standards of Good Clinical Practice (E6: Good Clinical Practice: Consolidated Guideline (CPMP/ICH/135/95) (D.M. 27 April 1992) and subsequent amendments (D.M., 15 July 1997 and D.Lvo 211/2003). The use of off-label drugs and the current study were registered with the local ethical committee.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We would like to thank Maria Vittoria Cossu for the necessary and strong help in conducting this study.
This research was supported by EU funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
This paper was edited by Parham Sendi and reviewed by three anonymous referees.
Azamgarhi, T., Warren, S., Scobie, A., Karunaharan, N., Perez-Sanchez, C., Houghton, R., Hassan, S., Lourtet-Hascoët, J., Kershaw, H., Sendi, P., and Saeed, K.: Dalbavancin to facilitate early discharge in the treatment of complex musculoskeletal infections: a multi-centre real-life application, J. Bone Joint Infect., 10, 93–100, https://doi.org/10.5194/jbji-10-93-2025, 2025.
Bork, J. T., Heil, E. L., Berry, S., Lopes, E., Davé, R., Gilliam, B. L., and Amoroso, A.: Dalbavancin Use in Vulnerable Patients Receiving Outpatient Parenteral Antibiotic Therapy for Invasive Gram-Positive Infections, Infect. Dis. Ther., 8, 171–184, https://doi.org/10.1007/s40121-019-0247-0, 2019.
Boucher, H. W., Wilcox, M., Talbot, G. H., Puttagunta, S., Das, A. F., and Dunne, M. W.: Once-Weekly Dalbavancin versus Daily Conventional Therapy for Skin Infection, New Engl. J. Med., 370, 2169–2179, https://doi.org/10.1056/nejmoa1310480, 2014.
Buckwalter, M. and Dowell, J. A.: Population Pharmacokinetic Analysis of Dalbavancin, a Novel Lipoglycopeptide, J. Clin. Pharmacol., 45, 1279–1287, https://doi.org/10.1177/0091270005280378, 2005.
Cattaneo, D., Fusi, M., Colaneri, M., Fusetti, C., Genovese, C., Giorgi, R., Matone, M., Merli, S., Petri, F., and Gori, A.: Therapeutic Drug Monitoring of Dalbavancin in Real Life: A Two-Year Experience, Antibiotics, 13, 20, https://doi.org/10.3390/antibiotics13010020, 2023.
Cattaneo, D., Fusi, M., Galli, L., Genovese, C., Giorgi, R., Matone, M., Merli, S., Colaneri, M., and Gori, A.: Proactive therapeutic monitoring of dalbavancin concentrations in the long-term management of chronic osteoarticular/ periprosthetic joint infections, Antimicrob. Agents Ch., 68, e00023-24, https://doi.org/10.1128/aac.00023-24, 2024.
Cojutti, P. G., Rinaldi, M., Gatti, M., Tedeschi, S., Viale, P., and Pea, F.: Usefulness of therapeutic drug monitoring in estimating the duration of dalbavancin optimal target attainment in staphylococcal osteoarticular infections: a proof-of-concept, Int. J. Antimicrob. Agents, 58, 106445, https://doi.org/10.1016/j.ijantimicag.2021.106445, 2021.
Dunne, M. W., Puttagunta, S., Sprenger, C. R., Rubino, C., Van Wart, S., and Baldassarre, J.: Extended-duration dosing and distribution of dalbavancin into bone and articular tissue, Antimicrob. Agents Ch., 59, 1849–1855, https://doi.org/10.1128/AAC.04550-14, 2015.
Dunne, M. W., Puttagunta, S., Giordano, P., Krievins, D., Zelasky, M., and Baldassarre, J.: A Randomized Clinical Trial of Single-Dose Versus Weekly Dalbavancin for Treatment of Acute Bacterial Skin and Skin Structure Infection, Clin. Infect. Dis., 62, 545–551, https://doi.org/10.1093/cid/civ982, 2016.
Esposito, S., Pagliano, P., De Simone, G., Guarino, A., Pan, A., Brambilla, P., Mastroianni, C., Lichtner, M., Brugnaro, P., Carretta, A., Santantonio, T., Brindicci, G., Carrega, G., Montagnani, F., Lapadula, G., Spolti, A., Luzzati, R., Schiaroli, E., Scaglione, V., Pallotto, C., Tacconi, D., Quintieri, F., and Trecarichi, E.: In-label, off-label prescription, efficacy and tolerability of dalbavancin: report from a National Registry, Infection, 52, 1297–1306, https://doi.org/10.1007/s15010-024-02176-2, 2024.
Gatti, M., Andreoni, M., Pea, F., and Viale, P.: Real-World Use of Dalbavancin in the Era of Empowerment of Outpatient Antimicrobial Treatment: A Careful Appraisal Beyond Approved Indications Focusing on Unmet Clinical Needs, Drug Des. Devel. Ther., 15, 3349–3378, https://doi.org/10.2147/DDDT.S313756, 2021.
Hanssen, J. L. J., van der Wal, R. J. P., van der Linden, H. M. J., van Prehn, J., Scheper, H., and de Boer, M. G. J.: Dosing and treatment duration of suppressive antimicrobial therapy in orthopedic implant infections: a cohort study, J. Bone Jt. Infect., 9, 149–159, https://doi.org/10.5194/jbji-9-149-2024, 2024.
Horne, M., Woolley, I., and Lau, J. S. Y.: The Use of Long-term Antibiotics for Suppression of Bacterial Infections, Clin. Infect. Dis., 79, 848–854, https://doi.org/10.1093/cid/ciae302, 2024.
Malabarba, A. and Goldstein, B. P.: Origin, structure, and activity in vitro and in vivo of dalbavancin, J. Antimicrob. Chemoth., 55, ii15–ii20, https://doi.org/10.1093/jac/dki005, 2005.
Martín, L. B., Fernández, M. M., Ruiz, J. M. P., Lafont, M. O., Paredes, L. Á., Rodríguez, M. Á. M., Regueras, M. F., Morón, M. Á. M., and Lobón, G. M.: Dalbavancin for treating prosthetic joint infections caused by Gram-positive bacteria: A proposal for a low dose strategy. A retrospective cohort study, Revista Espanola de Quimioterapia, 32, 532–538, 2019.
Mendes, R. E., Castanheira, M., Farrell, D. J., Flamm, R. K., Sader, H. S., and Jones, R. N.: Update on dalbavancin activity tested against Gram-positive clinical isolates responsible for documented skin and skin-structure infections in US and European hospitals (2011–13): Table 1, J. Antimicrob. Chemoth., 71, 276–278, https://doi.org/10.1093/jac/dkv303, 2016.
Morata, L., Cobo, J., Fernández-Sampedro, M., Guisado Vasco, P., Ruano, E., Lora-Tamayo, J., Sánchez Somolinos, M., González Ruano, P., Rico Nieto, A., Arnaiz, A., Estébanez Muñoz, M., Jiménez-Mejías, M. E., Lozano Serrano, A. B., Múñez, E., Rodriguez-Pardo, D., Argelich, R., Arroyo, A., Barbero, J. M., Cuadra, F., Del Arco, A., Del Toro, M. D., Guio, L., Jimenez-Beatty, D., Lois, N., Martin, O., Martínez Alvarez, R. M., Martinez-Marcos, F. J., Porras, L., Ramírez, M., Vergas García, J., and Soriano, A.: Safety and efficacy of prolonged use of dalbavancin in bone and joint infections, Antimicrob. Agents Ch., 63, 5, https://doi.org/10.1128/AAC.02280-18, 2019.
Rappo, U., Puttagunta, S., Shevchenko, V., Shevchenko, A., Jandourek, A., Gonzalez, P. L., Suen, A., Mas Casullo, V., Melnick, D., Miceli, R., Kovacevic, M., De Bock, G., and Dunne, M. W.: Dalbavancin for the Treatment of Osteomyelitis in Adult Patients: A Randomized Clinical Trial of Efficacy and Safety, Open Forum Infect. Dis., 6, ofy331, https://doi.org/10.1093/ofid/ofy331, 2019a.
Rappo, U., Dunne, M. W., Puttagunta, S., Baldassarre, J. S., Su, S., Desai-Krieger, D., and Inoue, M.: Epithelial Lining Fluid and Plasma Concentrations of Dalbavancin in Healthy Adults after a Single 1,500-Milligram Infusion, Antimicrob. Agents Ch., 63, 11, https://doi.org/10.1128/AAC.01024-19, 2019b.
Senneville, E., Cuervo, G., Gregoire, M., Hidalgo-Tenorio, C., Jehl, F., Miro, J. M., Seaton, A., Söderquist, B., Soriano, A., Thalhammer, F., and Pea, F.: Expert Opinion on Dose Regimen and Therapeutic Drug Monitoring for Long-Term Use of Dalbavancin: Expert Review Panel, Int. J. Antimicrob. Agents, 62, 106960, https://doi.org/10.1016/j.ijantimicag.2023.106960, 2023.
Silva, V., Miranda, C., Bezerra, M., Antão, H. S., Guimarães, J., Prada, J., Pires, I., Maltez, L., Pereira, J. E., Capelo, J. L., Igrejas, G., and Poeta, P.: Anti-biofilm activity of dalbavancin against methicillin-resistant Staphylococcus aureus (MRSA) isolated from human bone infection, J. Chemotherapy, 33, 469–475, https://doi.org/10.1080/1120009X.2021.1911518, 2021.
Söderquist, B.: Trough levels of dalbavancin during long-term treatment of prosthetic joint infections, in: EBJIS2024, 42nd Annual Meeting of the European Bone & Joint Infection Society, 26–28 September 2024, Barcelona, Spain, https://doi.org/10.1302/1358-992X.2024.19.022, 2024.
Weber, R. E., Fleige, C., Layer, F., Neumann, B., Kresken, M., and Werner, G.: Determination of a Tentative Epidemiological Cut-Off Value (ECOFF) for Dalbavancin and Enterococcus faecium, Antibiotics, 10, 915, https://doi.org/10.3390/antibiotics10080915, 2021.
Wouthuyzen-Bakker, M., Nijman, J. M., Kampinga, G. A., Assen, S. v., and Jutte, P. C.: Efficacy of Antibiotic Suppressive Therapy in Patients with a Prosthetic Joint Infection, J. Bone Joint Infect., 2, 77–83, https://doi.org/10.7150/jbji.17353, 2017.
Wunsch, S., Krause, R., Valentin, T., Prattes, J., Janata, O., Lenger, A., Bellmann-Weiler, R., Weiss, G., and Zollner-Schwetz, I.: Multicenter clinical experience of real life Dalbavancin use in gram-positive infections, Int. J. Infect. Dis., 81, 210–214, https://doi.org/10.1016/j.ijid.2019.02.013, 2019.